Introduction
Probiotics are live microbes that are beneficial to human health and require safety, gastrointestinal endurance, adhesion to intestinal cells, and other biological functions [1]. Its known effects include lowering cholesterol levels, improving gastrointestinal diseases, anticancer activity, blood pressure regulation, immune modulation, constipation relief, and allergy improvement [2].
Recent studies on dead cells have emerged and enhanced probiotic efficiency. Dead cells lose their bacterial viability through chemical or physical methods that disrupt their DNA and cell membranes [3]. Recent studies have highlighted the immunomodulatory functions of probiotics, dead cells, and their metabolites [4], with dead cells proving to be more stable and easier to use than probiotics [5].
Heat is commonly used method for non-viable preparations. Even after heat treatment, probiotic bacteria and their extracts maintain their probiotic properties in the intestine, ensuring safer preparation [6,7]. Heat inactivation conditions vary widely (5–60 min at 60°C–121°C), depending on the strain and expected benefits [3]. Lactobacillus and Bifidobacterium species, which are commonly used as heat-treated dead cells, exhibit diverse physiological activities and retain their functionality [8].
Dead cells possess antimicrobial, antioxidant, and immunomodulatory properties with physiological, immunological, neuro-hormonal, and metabolic effects [6]. Heat treatment decomposes the cell wall, facilitating antioxidant release [9], whereas sugars and proteins generate Maillard reaction products with antioxidant activity [10]. Heat-treated Lactobacillus strains suppress NO and cytokine production in lipopolysaccharide (LPS)-induced RAW 264.7 cells, confirming their immunomodulatory potential [11].
Lipoteichoic acid (LTA), a cell wall component of gram-positive bacteria, induces cytokine production (TNF-α, IL-1β, and IL-8) and affects gut microbiota, anti-aging, sepsis, and cholesterol levels [12]. Unlike LPS from gram-negative bacteria, LTAs from Lactobacillus strains exhibit diverse immunoregulatory effects. They promote immune responses by activating macrophages and spleen cells. LTA regulates cell wall autolysis and binds to immune receptors, enhancing immunity by, acting as an anti-inflammatory agent [13,14].
Despite the increasing interest in dead cells, research on LTA from gram-positive lactic acid bacteria (LAB) remains limited compared to that on pathogenic gram-positive bacteria [15]. Studies on the physiological effects of heat-treated dead cells are limited, particularly regarding LTA, which is a key component of dead cells. Furthermore, LTA content analysis during LAB heat treatment has yet to be reported. Therefore, in this study, LAB were heat-treated to prepare dead cells, their functional properties were compared with those of live cells, and their immunological properties were analyzed through cell experiments and LTA content measurements.
Materials and Methods
LAB were isolated from homemade kimchi collected from various regions of Korea. First, a 10 mL of kimchi sample was mixed with 40 mL of phosphate buffered saline (PBS; pH 7.4), homogenized and serially diluted with 0.85% Saline. Dillutions (100 μL) were plated onto De Man-Rogosa-Sharpe (MRS; BD Difco, USA) agar with 0.02% sodium azide and incubated at 37°C for 48 h. The LAB colonies were cultured in MRS broth and preserved at –70°C in MRS with 20% glycerol.
The selected strains were evaluated based on their colony shape, Gram staining results, and microscopic morphology. The identified strains were further analyzed using 16S rRNA gene sequencing, and their sequence homology was compared with 16S rRNA gene sequences available in GenBank (Macrogen, Korea).
Gastric juice and bile tolerance were evaluated as described by [16]. Selected strains were subjected to different stress conditions to assess their resistance. For the acid tolerance assay, cultures were introduced into MRS broth containing 0.3% pepsin, which had been adjusted to pH 2.0 using 1 N HCl, and subsequently incubated at 37°C for 2 h. After incubation at 37°C for 48 h, single colonies on MRS agar plates were examined. To determine bile tolerance, cultures were inoculated into MRS broth containing 0.3% oxgall and incubated at 37°C for 24 h. The survival rate was assessed using a previously described method.
LAB were subcultured three times at 16–18 h intervals in MRS broth, then harvested by centrifugation at 8,000×g for 10 min at 4°C. The supernatant was discarded and replaced with an equivalent volume of PBS (pH 7.4) and this washing process was repeated twice. The final bacterial concentration was adjusted to 1011 colony forming units (CFU)/mL. For the heat treatment, the cells were exposed to 65°C and 95°C for 5–60 min, and the viable cell count was determined. The survival rate was calculated using the following formula:
The heat-treated dead cells were centrifuged at 18,510×g for 10 min at 4°C, and the supernatant was collected for analysis. LTA analysis was performed using a general Lipoteichoic Acid ELISA kit (Mybiosource, USA) according to the manufacturer’s instructions, and absorbance was measured at 450±2 nm using a microplate reader (Thermo Fisher Scientific, USA).
The heat-treated dead cells were centrifuged at 18,510×g for 10 min at 4°C, and the supernatant was collected for analysis. Protein quantification was conducted using the Total Protein Assay kit (Abnova, Taiwan) according to manufacturer’s instruction, and absorbance was measured at 734 nm using a microplate reader.
The 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) diammonium salt) radical scavenging activity of live and dead cells was assessed following the method described by [17] with slight modifications. ABTS (Sigma-Aldrich, USA) was dissolved in distilled water (D.W.) to prepare a ABTS solution (7 mM). A potassium persulfate (Sigma-Aldrich) solution (2.45 mM) was prepared seperately. The ABTS solution and potassium persulfate were then mixed at a 1:1 (v/v) ratio and stored in the dark at room temperature for 12–16 h to allow radical formation. Following the incubation period, the ABTS reagent was diluted in PBS (pH 7.4) and adjusted until the absorbance was measured as 0.70±0.02 at 734 nm using a microplate reader. Subsequently, the ABTS reagent and live/dead cell samples were mixed at a 9:1 (v/v) ratio and incubated in the dark at room temperature for 6 min. After the reaction, the absorbance was measured at 734 nm, and the results were expressed in terms of the Trolox (Sigma-Aldrich) equivalent antioxidant activity (reported in mM).
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity of live and dead cell was evaluated according to the method described by [18] with some modifications DPPH radical solution (0.2 mM) was prepared for use in the experiment by mixing DPPH (Thermo Fisher Scientific) and ethanol (Samchun Pure Chemical, Korea). Next, the DPPH solution and live/dead cell samples were mixed in a 1:1 ratio (v/v) and incubated at room temperature in the dark for 30 min. The absorbance of the solution was measured at 517 nm, and the results were expressed in units of ascorbic acid (Sigma-Aldrich) equivalent capacity in millimolar (mM).
The ferric reducing antioxidant power (FRAP) of live and dead cells was determined using the method described by [19] with slight modifications. An acetate buffer solution (300 mM) was prepared by dissolving sodium acetate trihydrate (Sigma-Aldrich) in acetic acid (Samchun Pure Chemical). A HCl solution (40 mM) was prepared by mixing HCl (Samchun Pure Chemical) in D.W., whereas a 2,4,6-tri(2-pyridyl)-1,3,5-triazine (TPTZ) solution (10 mM) was prepared by dissolving TPTZ (Sigma-Aldrich) in D.W. Additionally, a 20 mM iron (III) chloride hexahydrate solution was prepared by dissolving 97% iron (III) chloride hexahydrate (A.C.S reagent; Sigma-Aldrich) in ethanol (Samchun Pure Chemical). To prepare the TPTZ reagent, 40 mM HCl and 10 mM TPTZ were mixed at a 1:1 (v/v) ratio. Then, the acetate buffer solution, TPTZ reagent, and iron (III) chloride hexahydrate solution were then combined in a 10:1:1 (v/v) ratio to create the FRAP working solution, which was pre-warmed at 37°C before use. For the assay, the FRAP solution and live/dead cell samples were mixed at a 19:1 (v/v) ratio and allowed to react in the dark at room temperature for 30 min. After incubation, absorbance was recorded at 593 nm, and the results were expressed in terms of ascorbic acid (Sigma-Aldrich) equivalent capacity (mM).
RAW 264.7 cells (KCTC, Korea) were maintained in Dulbecco’s Modified Eagle’s Medium (Welgene, Korea) supplemented with 10% heat-inactivated fetal bovine serum (Sigma-Aldrich). Cultures were incubated at 37°C in a 5% CO2 environment to ensure optimal growth conditions.
RAW 264.7 cells were seeded into a 96-well cell culture plate at a density of 1×105 cells/well and incubated for 16–18 h for adhesion. After removing the medium, live/dead cell sample (100 μL) was added and incubated at 37°C with 5% CO2 for 20–22 h. After incubation, the medium was removed, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich) solution (100 μL) was added to each well to a final concentration of 5 mg/mL. The plate was further incubated at 37°C in a 5% CO2 incubator for 2–3 h to allow formazan crystal formation. Once the reaction was complete, the medium was discarded, and 100 μL of dimethyl sulfoxide (Biosesang, Korea) was added to each well to fully dissolve the purple formazan crystals formed by the reduction reaction. The absorbance was then measured at 540 nm using following the method described by [20].
NO levels were determined by measuring nitrite in the cell culture supernatant using the griess reagent assay. RAW 264.7 cells were seeded into a 96-well cell culture plate at a density of 1×105 cells/well and incubated for 16–18 h. After removing the medium, live/dead cell samples at various concentrations were added. Thereafter each sample (100 μL) was dispensed into the wells and incubated at 37°C with 5% CO2 for 2–3 h. Subsequently, 100 μL of LPS (Sigma-Aldrich) at a final concentration of 100 ng/mL was introduced into each well, and the plate was further incubated for 20–22 h. After incubation, cell-free supernatant (100 μL) was transferred to a new plate, mixed with an equal volume of Griess reagent, and incubated in the dark for 15 min absorbance was measured at 540 nm using a microplate reader.
The cytokine content in the supernatant of RAW 264.7 cell cultures was measured using a mouse TNF-α, IL-1α, and IL-6 ELISA kit (Komabiotech, Korea) following the manufacturer’s instructions, and absorbance was measured at 450 nm using a microplate reader.
The LTA extraction and high-performance liquid chromatography (HPLC) analysis were conducted following the method described by [21] with slight modifications. Heat-treated dead cells (200 mL) were centrifuged at 18,510×g for 20 min at 4°C and resuspended twice in 20 mL of 0.1 M sodium citrate (pH 4.7; Sigma-Aldrich) and sonicated at 400 W for 5 min. After centrifugation, the pellet was extracted using 10 mL of 0.1 M sodium citrate and 10 mL of 1-butanol, shaken at room temperature for 30 min and centrifuged. The lower aqueous phase was collected and evaporated using a rotary evaporator, resuspended in 10 mL of D.W. and lyophilized. For HPLC analysis, 10 mg of lyophilized LTA sample was dissolved in 0.5 mL of 45% 1-propanol in 0.1 M ammonium acetate (pH 4.7; Sigma-Aldrich), centrifuged at 17,000×g for 60 min, and analyzed by reverse-phase (RP)-HPLC (Waters, USA) on a C8 column (Symmetry® C8, 3.9 mm×150 mm, particle size 5 μm, Waters) with a mobile phase of 45% 1-propanol in 0.1 M ammonium acetate (pH 4.7) (Table 1).
Results
After 48 h of incubation, colonies of LAB were observed on the MRS agar plates. Five strains were isolated from kimchi samples and preserved at –70°C in a mixture of 20% glycerol and MRS broth for long-term storage.
For the Gram staining assay, the morphological characteristics of the isolated strains were examined under a microscope, which revealed that all strains were gram-positive. Additionally, 16s rRNA gene sequencing was performed to identify five strains, belonging to various Lactobacillus species. The 16s rRNA gene sequences of these strains are listed in Table 2.
Tolerance of the isolated strains to artificial gastric juice and bile salts was evaluated under simulated gastrointestinal conditions. Since the human stomach typically processes food within 20–120 min [22], the viability of LAB was tested after 2 h exposure to environments with pH 1.5, 2.0, and 3.0 at 37°C, followed by plate counting using BCP agar. The results demonstrated that LAB exhibited strong resistance to simulated gastric juice conditions (Table 3), consistent with findings by [23], which indicated that probiotic strain tolerance is strain dependent. Additionally, bacterial resistance to intestinal bile salts was assessed, because bile tolerance is a crucial factor in probiotic selection. Previous studies [24,25] have reported that human intestinal bile oxgall concentrations typically range from 0.3%–0.5%. To mimic these conditions, an artificial digestive environment was created, with oxgall concentrations of 0.3%, and the number of viable bacteria was determined. These strains demonstrated good resistance to artificial bile juice (Table 4), supporting findings of previous studies [25,26] that highlighted strain-specific variations in LAB survival rates under artificial bile concentrations.
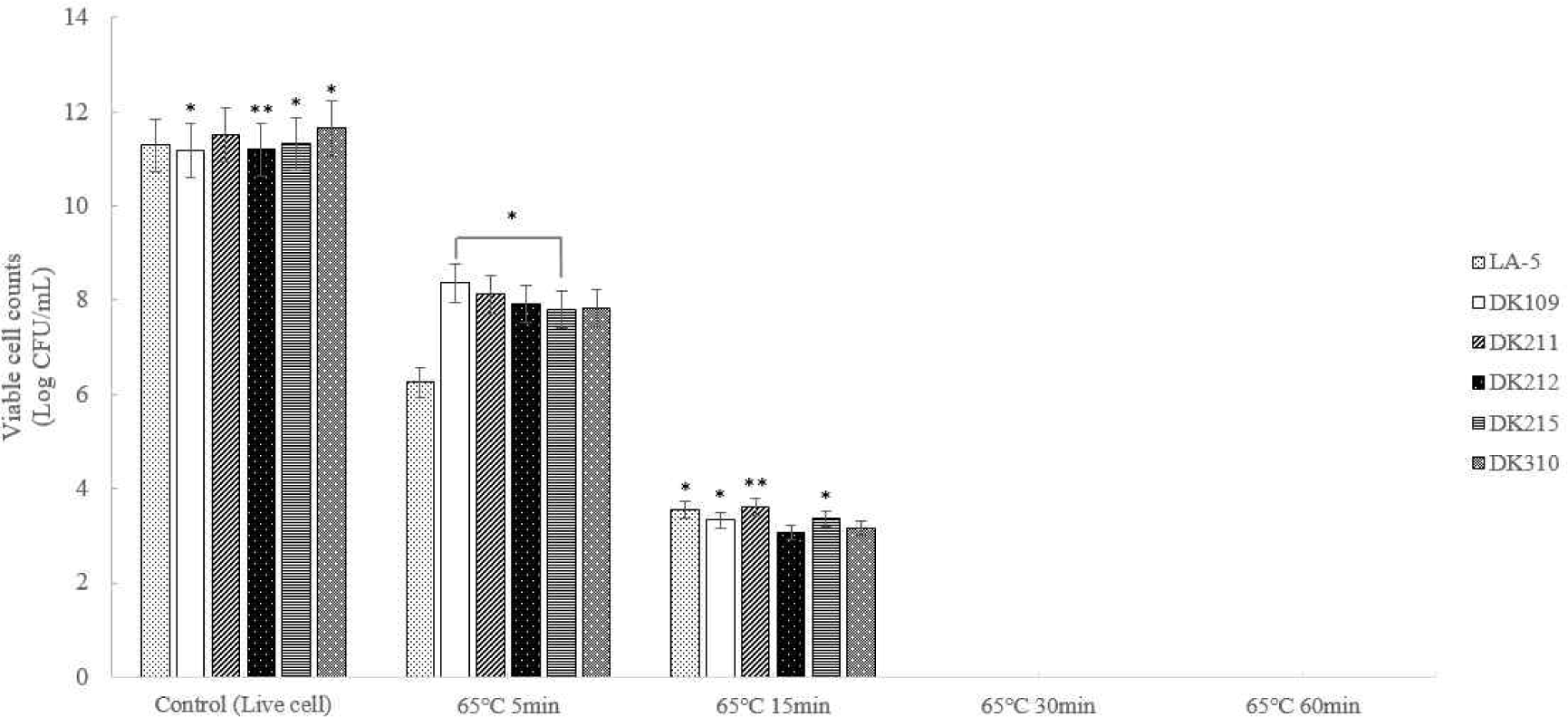
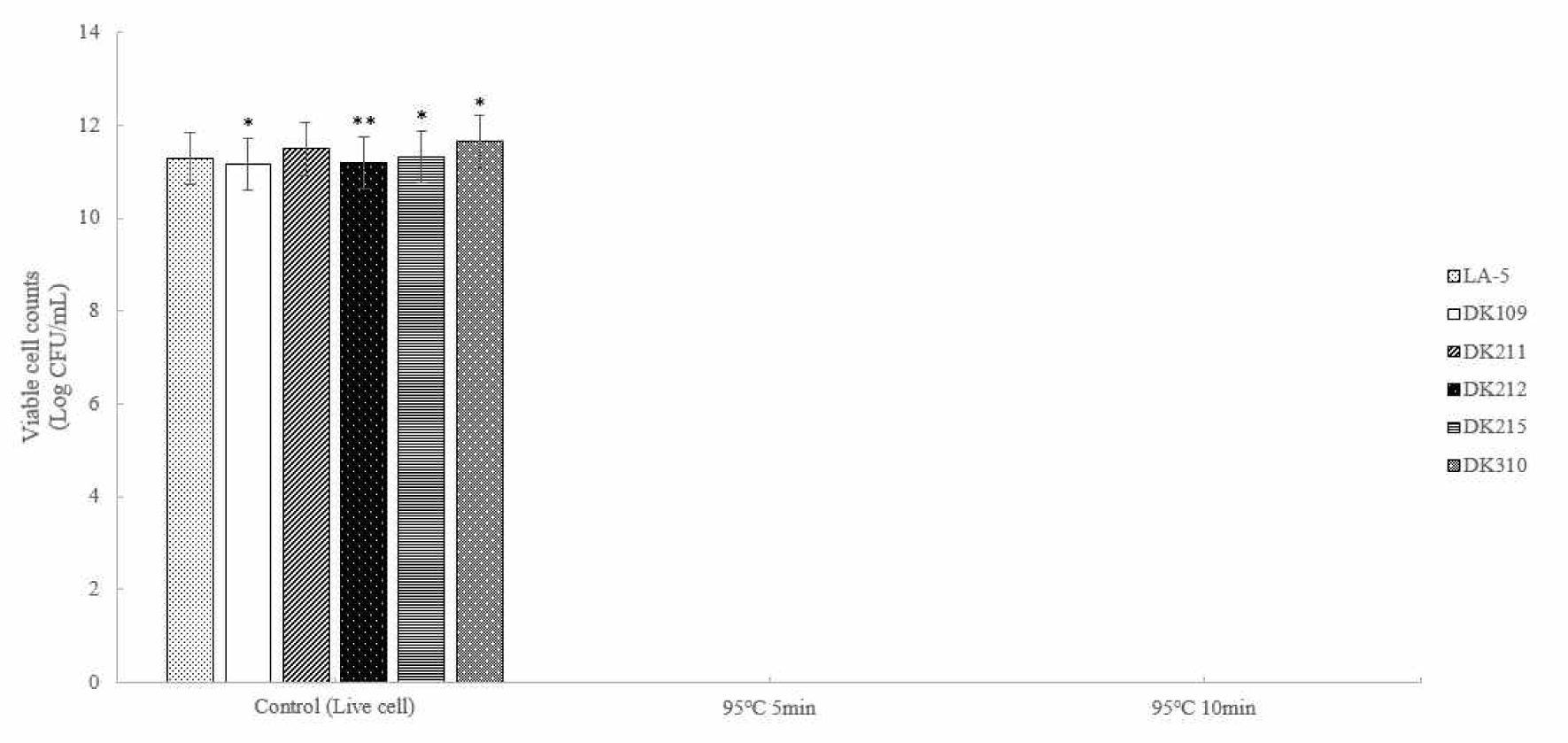
Five strains of lactic acid bacteria (LAB) were heat-treated at 65°C for 5, 15, 30, and 60 min and at 95°C for 5 min and 10 min to induce bacterial inactivation. Thereafter the number of viable bacteria was determined (Figs. 1 and 2). The results confirmed that complete inactivation occurred at 65°C for 30 min or longer and at 95°C for 5 min or longer.
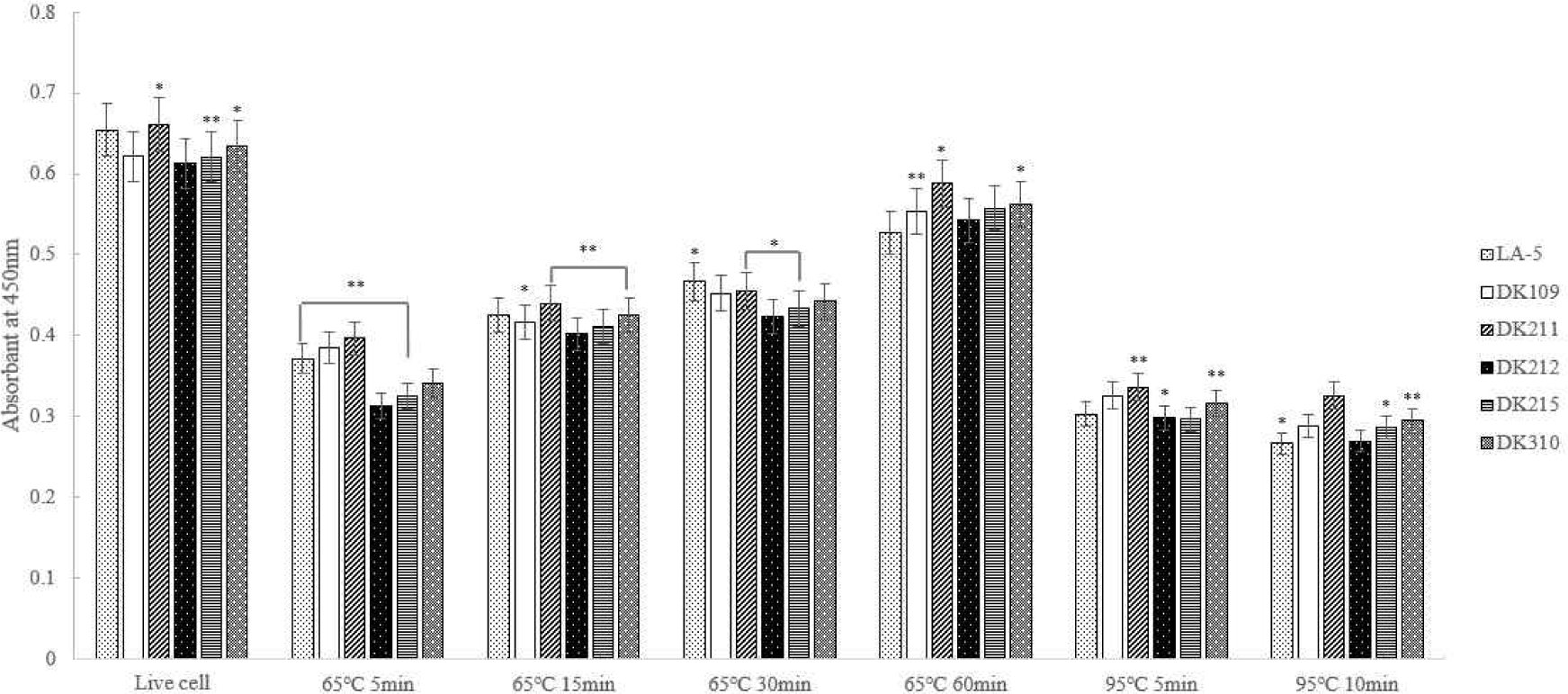
The experimental results showed that LTA expression was highest at 65°C for 60 min. Additionally, DK211, DK215, and DK310 exhibited higher LTA expression rates under treatment at 65°C for 60 min and 95°C for 10 min compared to the commercial strain LA-5 (Fig. 3).
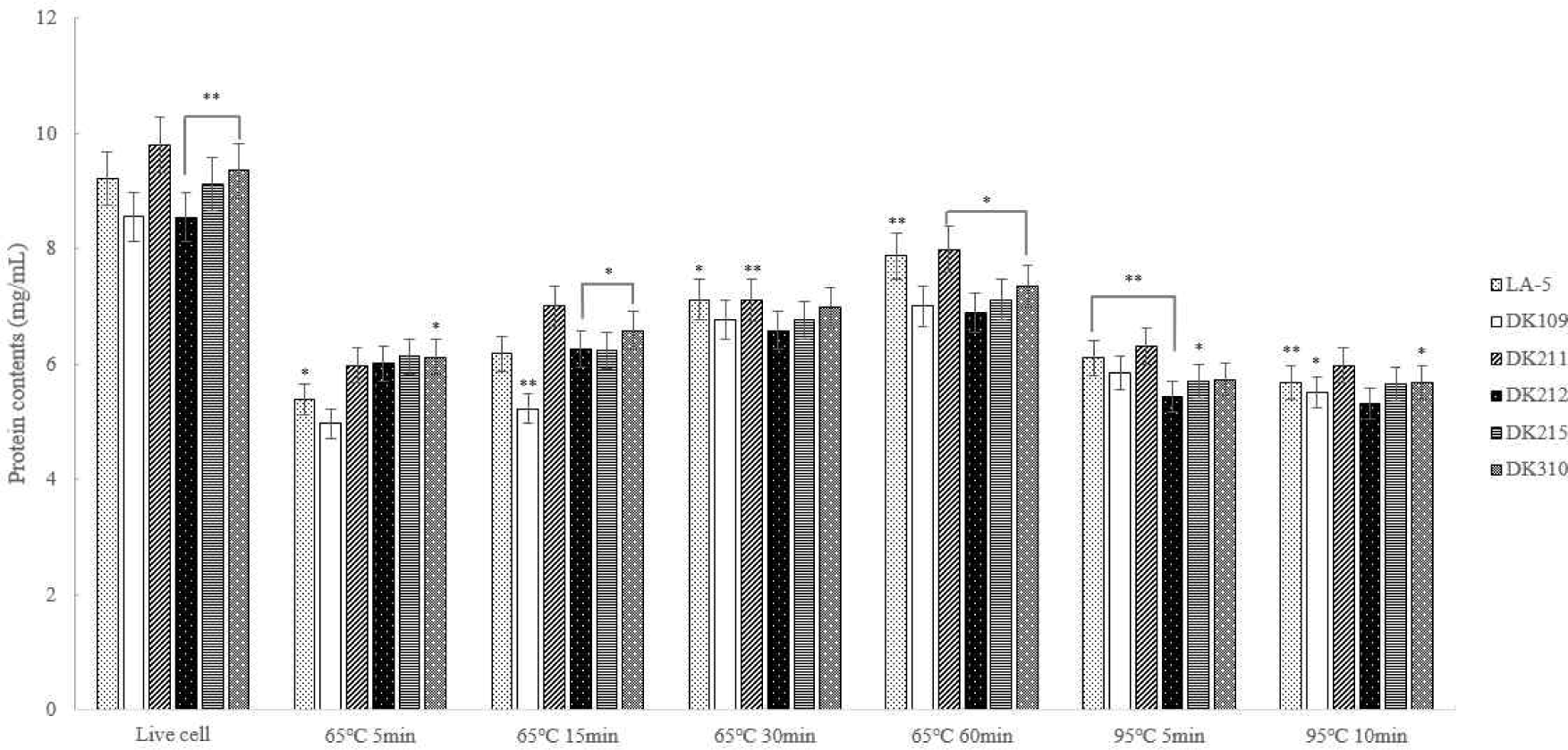
The quantitative protein analysis revealed that protein content in dead cells heat-treated at 95°C was lower than in those heat-treated at 65°C. This suggests that heat treatment at 95°C led to protein degradation, resulting in reduction in protein content (Fig. 4).
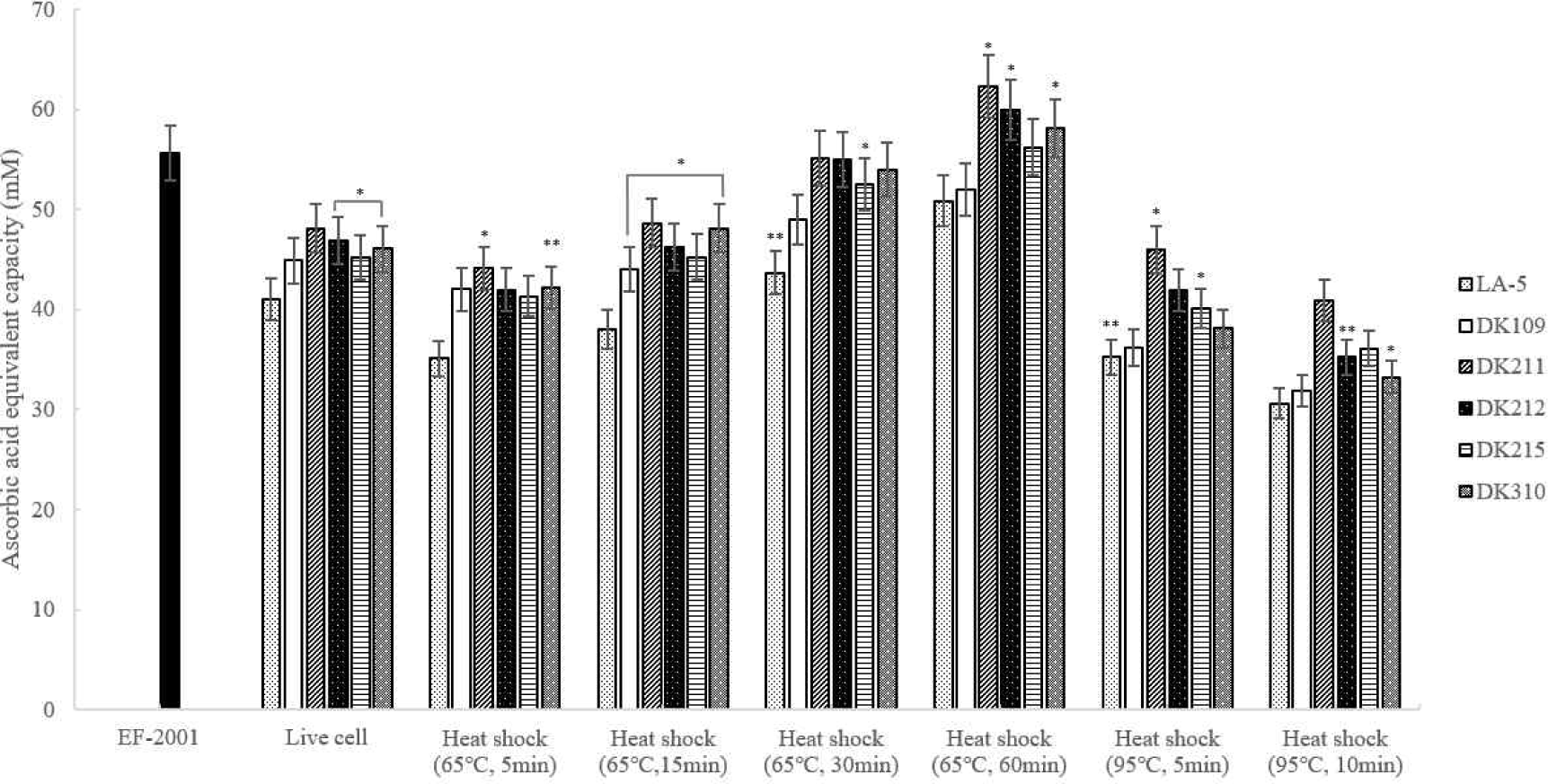
ABTS radical scavenging activity was measured and heat-treated DK211 and DK310 exhibited higher antioxidant activity than both LA-5 and live cells. Among the tested strains, DK211 and DK310 demonstrated greater ABTS radical scavenging activity under the same heat-treatment conditions. In particular, DK211 heat-treated at 65°C for 60 min demonstrated higher ABTS radical scavenging activity than Enterococcus faecalis (EF-2001), a commercially available heat-killed probiotc strain (Fig. 5).
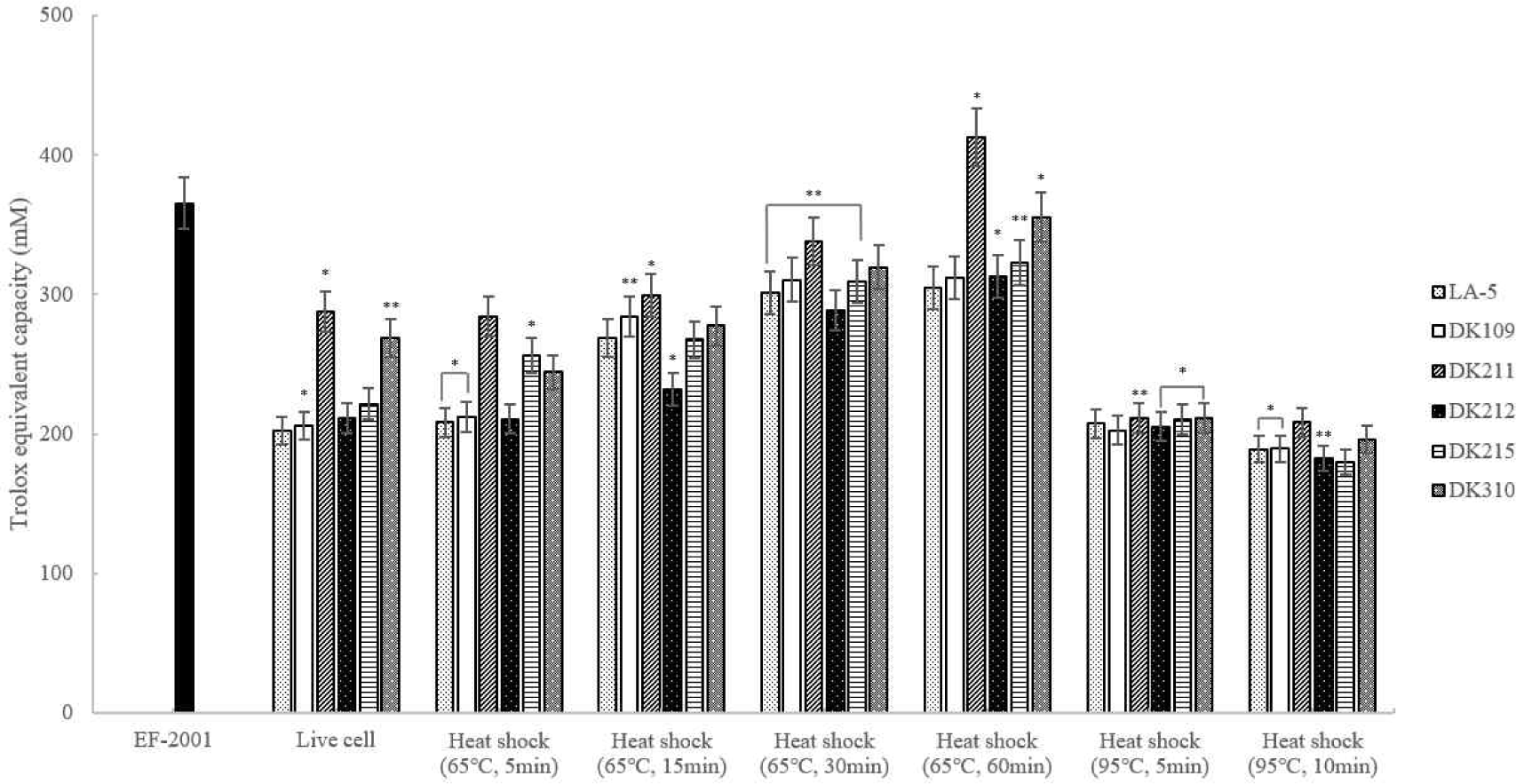
The DPPH assay was used to evaluate the antioxidant activity of heat-treated LAB strains. As shown in Fig. 6, dead cells heat-treated at 65°C for 30 and 60 min exhibited antioxidant activity comparable to or higher than that of live cells. Notably, DK211 and DK310 heat-treated at 65°C for 60 min demonstrated greater DPPH radical scavenging activity than EF-2001, a commercially available heat-killed probiotic strain.
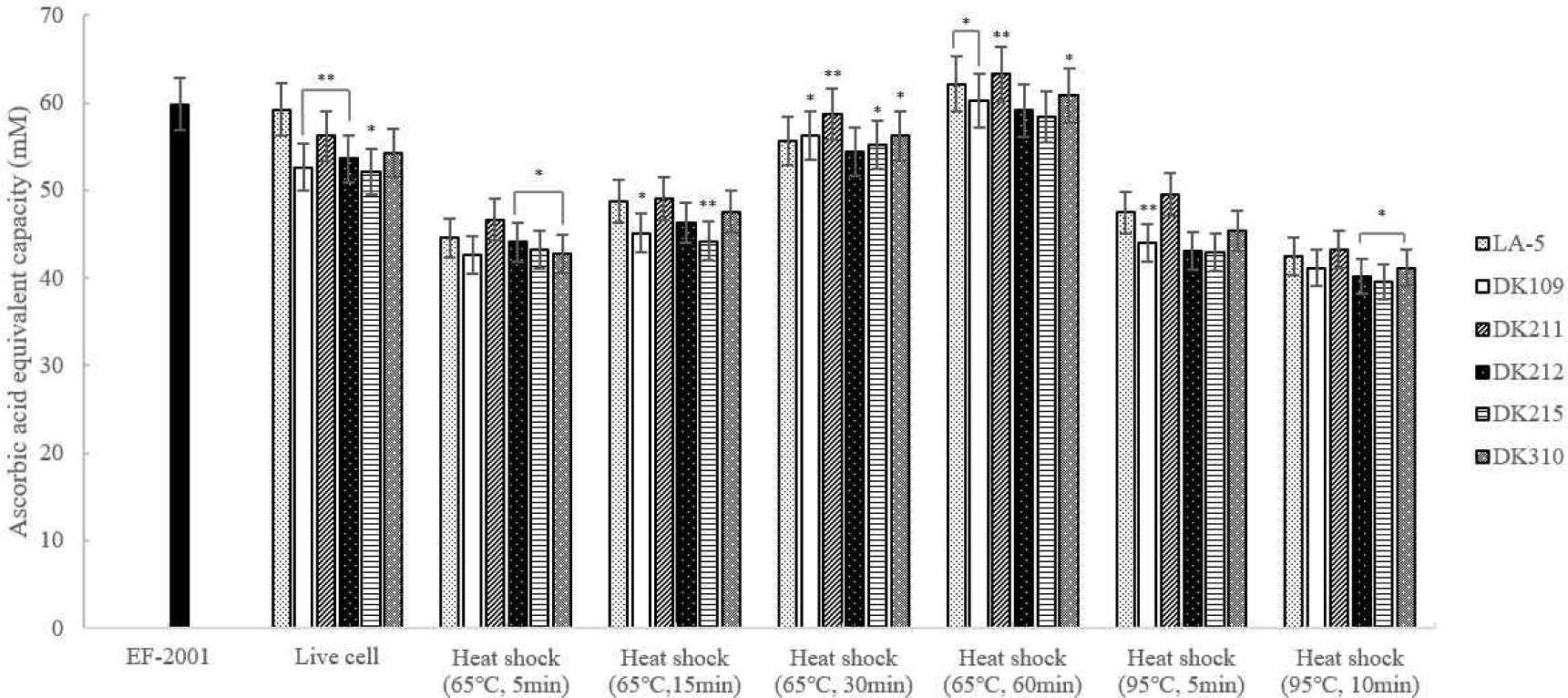
The FRAP assay was used to assess the antioxidant activity of heat-treated LAB strains by measuring their electron donation ability. As shown in Fig. 7, all DK strains exhibited higher iron-reducing capacity than LA-5 under the same heat-treatment conditions. Notably, DK211, DK212, and DK310 heat-treated at 65°C for 60 min demonstrated significantly greater FRAP activity than EF-2001, a commercially available heat-killed probiotic strain. Based on these findings, 65°C for 60 min was selected as the optimal heat treatment condition for further evaluation of anti-inflammatory effects.
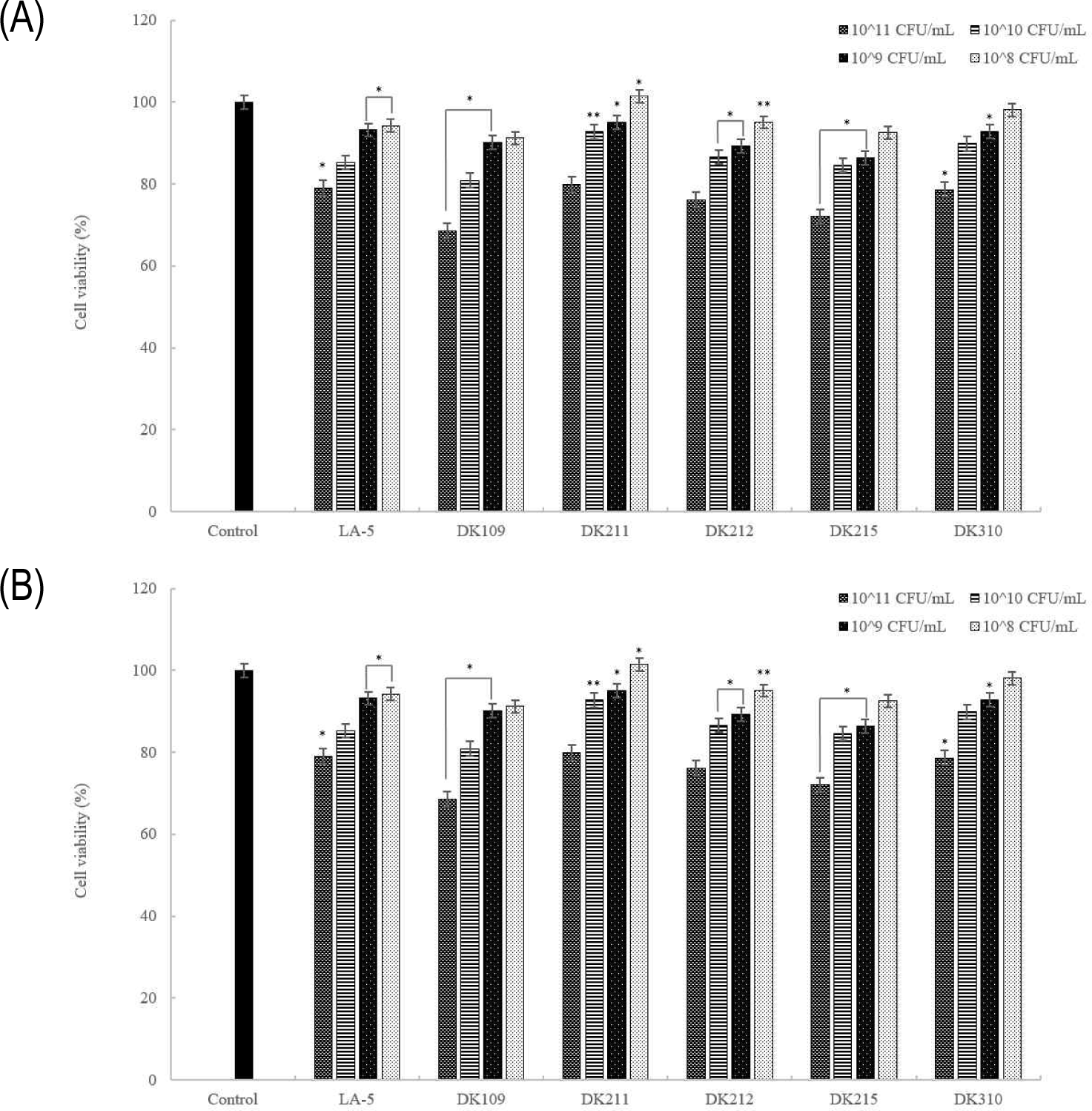
The MTT assay was used to assess the viability of RAW 264.7 macrophages after treatment with live and heat-treated LAB cells. As shown in Fig. 8, live cells exhibited no cytotoxicity or growth-promoting effects at any concentration except 1011 CFU/mL, while dead cells showed no cytotoxic effects across all tested concentrations. Notably, both live and dead cells maintained over 80% viability, confirming that they do not induce cytotoxic effects in macrophages.
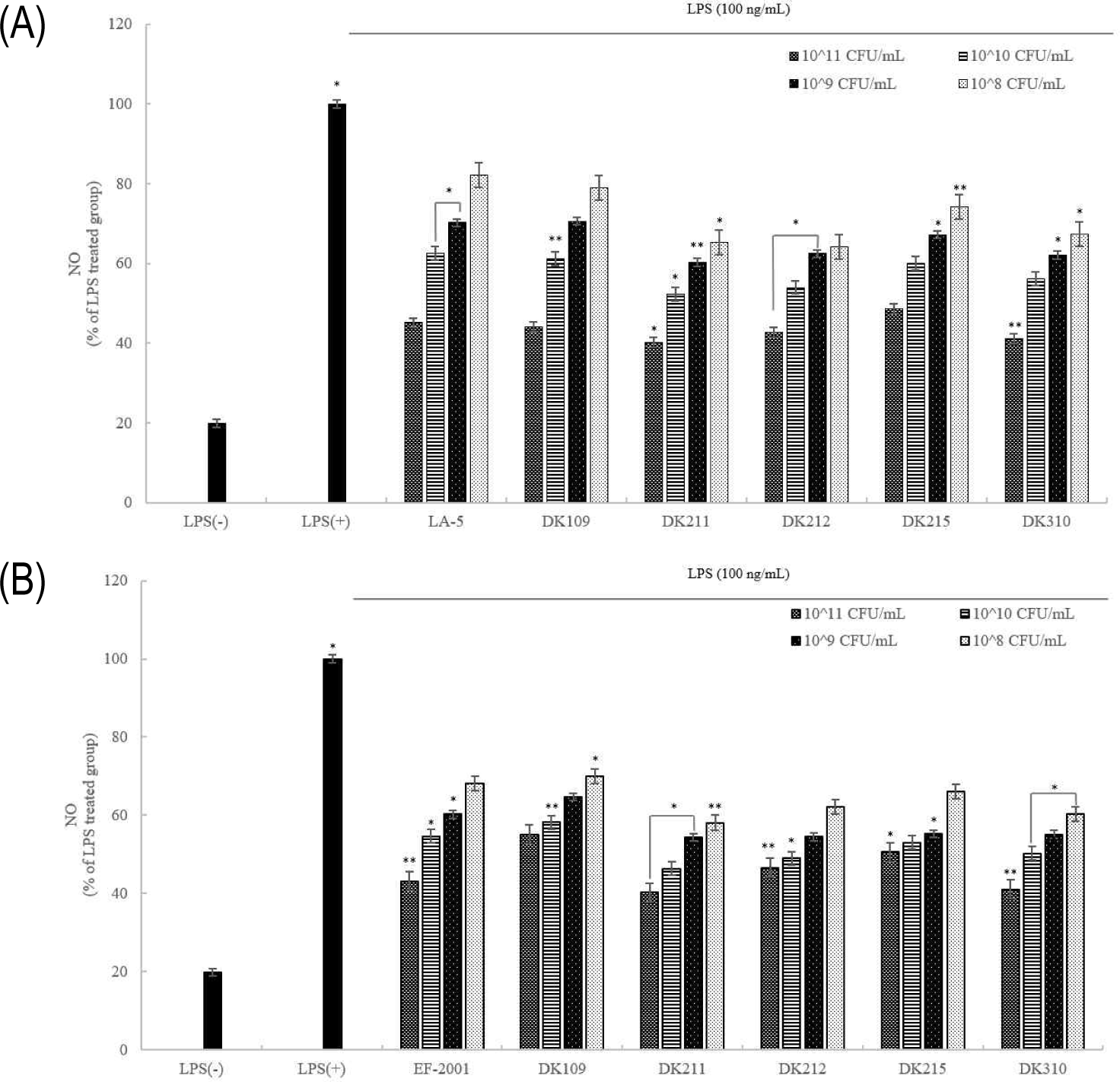
NO assay was performed to evaluate the anti-inflammatory effects of heat-treated LAB strains in LPS-stimulated RAW 264.7 macrophages. As shown in Fig. 9, LPS treatment significantly increased NO production, whereas treatment with both live and dead LAB cells effectively suppressed NO production in a concentration-dependent manner. These findings suggest that heat-treated LAB strains exhibit anti-inflammatory potential by reducing excessive NO production associated with inflammation.
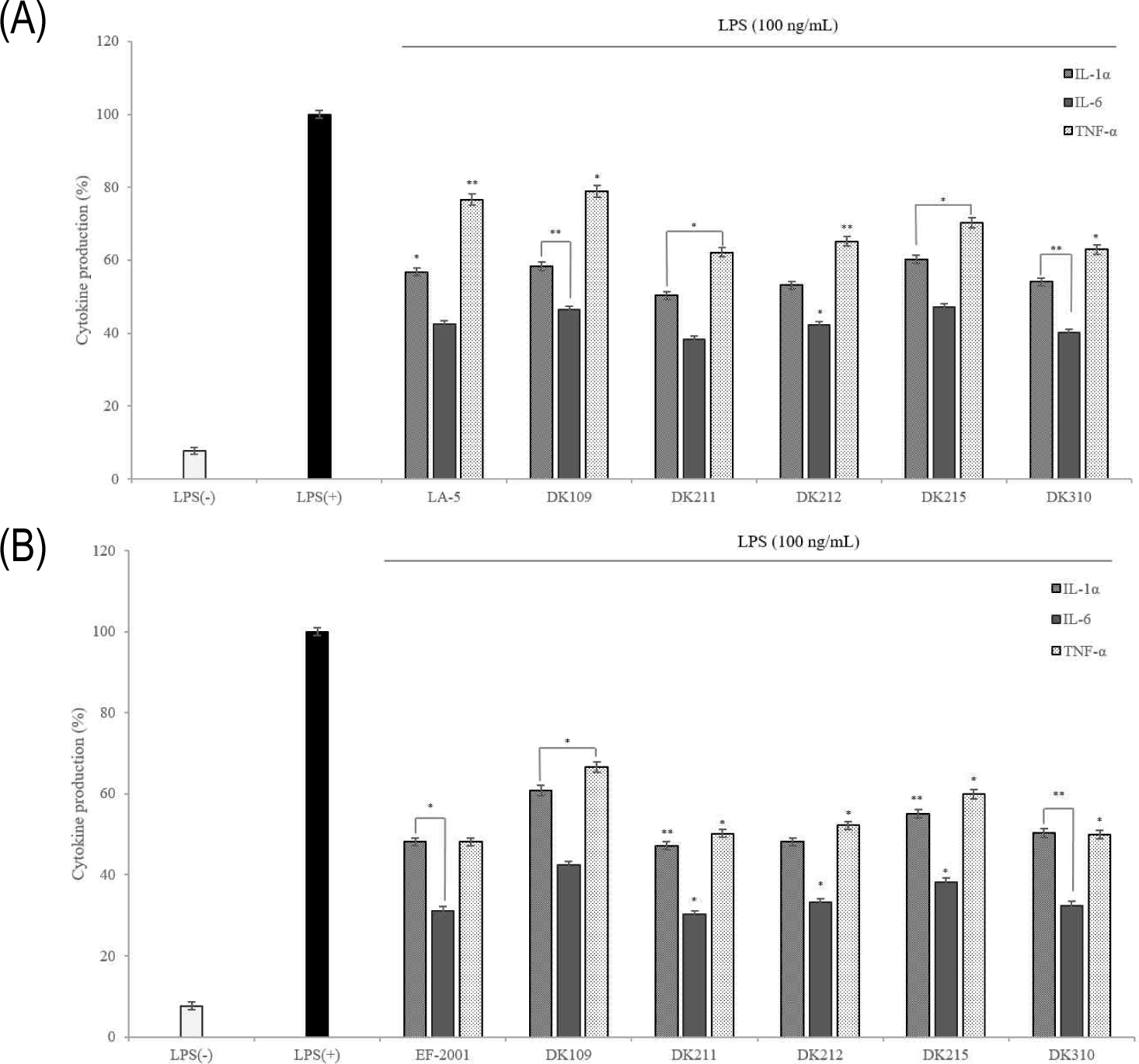
Maintaining the immune balance requires direct and indirect interactions between immune cells, with cytokines playing crucial roles in the proliferation, differentiation, and functional regulation of immune responses. Many diseases are associated with inflammation, where inflammatory cells secrete inflammatory cytokines in response to harmful environmental factors, viruses, and drugs [27]. In this study, the expression on levels of TNF-α, IL-1α, and IL-6 were measured using ELISA, and results showed that their production was significantly lower in the treated live and dead cells than in the LPS (+) control group (Fig. 10). Additionally, dead cells exhibited lower TNF-α levels than live cells, suggesting that their effect on macrophages may help prevent the activation of pro-inflammatory cytokines induced by TNF-α expression. These findings indicate that both live and dead cells have the potential to exert anti-inflammatory effects.
LTA is a cell wall component of gram-positive bacteria, although numerous studies have been conducted, most studies have focused on pathogenic gram-positive bacteria, with limited investigations into LTA in LAB. In the present study, LTA was extracted using butanol and analyzed using a Symmetry C8 column in a RP-HPLC system. The LTA content in dead cells was quantified, and it was revealed that the commercial strain EF-2001 contained 2.67 mg/mL, whereas DK211 and DK310 contained 3.74 mg/mL and 3.72 mg/mL, respectively (Table 5). These findings confirmed that DK211 and DK310 had higher LTA content than the commercial strain EF-2001.
| Sample | LTA content (mg/mL) |
|---|---|
| EF-2001 | 2.67 |
| Dead cell DK211 | 3.74 |
| Dead cell DK310 | 3.72 |
Discussion
This study examined the functional and immune effects of live and heat-treated dead LAB cells by analyzing their properties under different heat treatment conditions. Five kimchi-derived LAB strains were identified as one Lactiplantibacillus plantarum strain, two Lacticaseibacillus casei strains, and two Lacticaseibacillus paracasei strains, selected based on their physiological activity. All strains demonstrated strong resistance to human digestive enzymes, maintaining stability at pH 1.5 and tolerating artificial gastric juice and bile salts.
The bacteria were completely inactivated at 65°C for 30 min and 95°C for more than 5 min. LTA expression was analyzed in heat-treated LAB lysates, revealing that LTA expression increased with longer heat treatment durations, with DK211 and DK310 exhibiting the highest levels. Protein content analysis showed that LTA expression at 95°C was lower than at 65°C after 60 min, likely due to protein degradation at higher temperatures. Similarly, protein content was highest at 65°C for 60 min, confirming that excessive heat leads to protein breakdown and reduced LTA expression.
Antioxidant activity varied by assay but consistently showed that heat-treated cells at 65°C for 60 min had stronger activity than live cells. DK211, DK212, and DK310 exhibited greater antioxidant activity compared with EF-2001 in ABTS, DPPH, and FRAP assays. Heat-treated cells contain bioactive components such as intracellular proteins, polysaccharides, peptides, and polyphenols, which have been shown to contribute to the scavenging of reactive oxygen species (ROS) and thereby exert antioxidant effects [28]. In addition, heat treatment facilitates the release of various bioactive molecules, including exopolysaccharides, peptidoglycans, and LTA, which are presumed to play an important role in enhancing antioxidant capacity [7,29–31].
Among these components, LTA has received considerable attention as a key determinant of both antioxidant and immunomodulatory activity. LTA from pathogenic bacteria, such as Staphylococcus aureus, is known to activate Toll-like receptor 2 (TLR2)-mediated NF-κB signaling, thereby upregulating pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, and simultaneously promoting ROS generation, inflammation, and cellular damage [32,33]. Furthermore, LTA stimulation in macrophages and microglial cells has been reported to increase NO production and inducible nitric oxide synthase expression, while reducing the activity of antioxidant enzymes [34].
Conversely, LTA derived from probiotic Lactobacillus strains exhibits potent anti-inflammatory and antioxidant activities. For example, in a mouse model, administration of LTA purified from L. paracasei significantly decreased oxidative stress markers such as myeloperoxidase, NO, and malondialdehyde, while simultaneously increasing the levels of major antioxidant factors, including total superoxide dismutase and reduced glutathione, thereby enhancing overall antioxidant capacity [35]. Similarly, β-carotene bleaching assays confirmed antioxidant potential, as Heat treated L. plantarum exhibited strong activities, underscoring their potential as functional paraprobiotics [36].
In line with these reports, our results also demonstrated that heat-treated LAB cells downregulated cytokine production in LPS-induced macrophages. Expression of TNF-α, IL-1β, and IL-6 was significantly decreased compared with the control strain, consistent with observations that heat treatment modulates cytokine production depending on the amount of LTA released [37]. Furthermore, LTAs isolated from three different Lactobacillus strains exhibited distinct regulatory effects in RAW 264.7 cells, increasing anti-inflammatory IL-10 secretion while suppressing TNF-α and IL-6 expression, consistent with previous reports [38].
Taken together, these findings indicate that the immunomodulatory effects of LTA derived from Lactobacillus strains is not uniform but strain-specific, reflecting structural heterogeneity. Multi-spectrometric analyses of L. plantarum LTAs confirmed clear strain-dependent variability in chemical composition and architecture, providing a mechanistic explanation for divergent immunological functions [39]. Importantly, this supports the hypothesis that LTA structure influences the interaction between microbe-associated molecular patterns and host pattern recognition receptors, thereby modulating downstream cytokine expression via NF-κB and MAPK signaling cascades.
The commercial strain E. faecalis EF-2001 has previously been recognized for its immunomodulatory properties, including anti-inflammatory and anti-allergic effects [40]. In our study, DK211 and DK310 not only showed comparable functional effects but also contained higher levels of LTA compared to EF-2001, suggesting their potential as candidate paraprobiotics. Since LTA bioactivity varies widely depending on bacterial species [41,42], further studies should aim to establish standardized purification protocols and reliable quantitative methods, along with in vivo validation models for their functional activities.
Collectively, our study highlights the potential of heat-treated LAB, particularly DK211 and DK310, as promising paraprobiotics with enhanced antioxidant and immunomodulatory properties. Notably, analysis of LTA extracted from heat-treated Lactobacillus strains (DK211, DK310) and Enterococcus faecalis EF-2001 via RP-HPLC revealed higher LTA levels in DK211 and DK310 compared to EF-2001. These findings, together with previous reports on the antioxidant and anti-inflammatory properties of LTA, indirectly suggest that dead cells may exhibit greater functional activity than live cells. However, further in vivo investigations are needed to confirm their efficacy and to elucidate the precise molecular mechanisms of LAB-derived LTA in immune regulation.






