SHORT COMMUNICATION
Production of Casein Hydrolysates from Concentrated Skim Milk Using Ultrafiltration Techniques
Hee Song Kim1
,
Dong Hun Yang1
,
Seok Jun Park2
,
Hye Jin Kim2
,
Hyoung Su Park2
,
Eui-Jong Lee3
,
Mee-Ryung Lee1,*
Author Information & Copyright ▼
1Department of Food and Nutrition, Daegu University, Gyeongsan, Korea
2R&D Group, Maeil Health Nutrition Co., Ltd., Pyeongtaek, Korea
3Department of Environmental Engineering, Daegu University, Gyeongsan, Korea
*Corresponding author : Mee-Ryung Lee, Department of Food and Nutrition, Daegu University, Gyeongsan, Korea, Tel : +82-53-850-6837, Fax : +82-53-850-6839, E-mail :
mrlee@daegu.ac.kr
© Copyright 2023, Korean Society of Dairy Science and Biotechnology. This is an Open Access article distributed under the terms of the
Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits
unrestricted non-commercial use, distribution, and reproduction in any
medium, provided the original work is properly cited.
Received: Sep 17, 2023; Revised: Sep 22, 2023; Accepted: Sep 22, 2023
Published Online: Sep 30, 2023
Abstract
Milk protein is often fractionated/concentrated by using various techniques in dairy industries. Among these techniques, ultrafiltration (UF) is particularly efficient at concentrating the casein fraction of milk protein. The objectives of this study were to produce casein hydrolysates by concentrating the casein fraction in skim milk using the UF technique and to investigate the chemical composition of the casein hydrolysates. The skim milk was concentrated using a UF laboratory test unit equipped with 10 kDa and 30 kDa membranes. After UF, the protein content of the milk was concentrated up to ∼7.2% and the Ca was concentrated up to ∼196 mg/100 g of milk. Trypsin was then added to the concentrated skim milk to produce the casein hydrolysates. The results of sodium dodecyl sulphate-polyacrylamide gel electrophoresis showed that the casein fraction was not present after hydrolysis, indicating that casein in the milk had been hydrolyzed. The Ca content in the casein hydrolysates was much higher (p<0.05) compared to Ca content in commercial casein phosphopeptides (CPP) indicating that was acidified during the manufacture of commercial CPP. In conclusion, it seems that casein hydrolysates containing large concentrations of protein and Ca can also be made from concentrated UF milk without acidification or renneting.
Keywords: ultrafiltration; concentrated skim milk; casein hydrolysates
Introduction
Bovine milk contains about 3.4% of protein of which ∼80% is casein and ∼20% is whey protein [1]. Casein hydrolysates, such as casein phosphopeptides (CPP), are commercially available to be used as a micronutrient supplement in a variety of functional foods, dietary supplements, and infant formula. CPP can promote the absorption and utilization of trace elements such as calcium, iron, and zinc, mainly because of its three serine phosphate clusters and two glutamic acid residues [2].
Ultrafiltration (UF) is a membrane filtration process that separates components based on molecular weights. Based on the pore size of the membrane, relatively higher molecular weight materials, such as protein and fat are retained and relatively lower molecular weight material such as lactose, minerals, other solutes, and water are permeated, respectively [3]. Commercial casein hydrolysates, such as CPP, are usually produced by enzymatic treatment of acid-precipitated or renneted milk, along with precipitation with barium or calcium ions following the removal of the bitter peptides with active carbon [4]. During this process, milk casein can be chemically modified with acidification. CPP prepared from unmodified casein micelles can contain higher amount of calcium as Ca is easily dissolved daring acidification of milk [5].
In South Korea, the uses of milk protein powders in a variety of food products such as infant and toddler formula, nutritional supplements for adults and sports products have been dramatically increased recently. According to the Food Information Statistics System (aT FIS), the proportion of powdery forms of milk used in 2021 was 33% for skim milk powder and 17% for whole milk powder, respectively. However, most milk protein powders used in South Korea are mostly from overseas despites of excess production of domestic bovine milk [6]. Therefore, the objectives of this study were to produce the casein hydrolysates from concentrated domestic skim milk using UF techniques without acidification or renneting of milk and to investigate the chemical properties of casein hydrolysates from ultrafiltered milk.
Materials and Methods
1. Preparation of concentrated milk with ultrafiltration (UF)
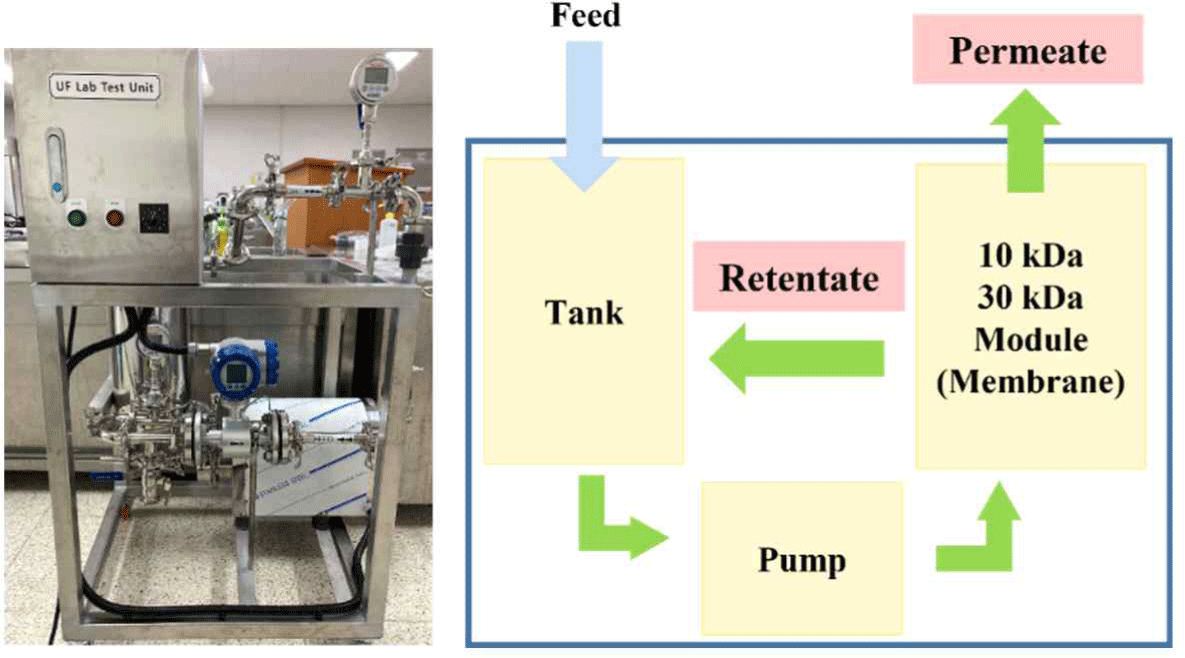
To obtain concentrated milk, 5 L of sterilized non-fat milk (Maeil Dairies, Korea) was concentrated using an UF Lab test unit (Pure-Envitech, Korea; Fig. 1) using membrane of 10 kDa and 30 kDa. The flow rate was kept constant from 1.45 to 1.6 m/s and concentration was performed in time (hr) and volume concentration factor (VCF) units. VCF was calculated by following equation (1):
2. Composition of concentrated milk with ultrafiltration (UF) and casein hydrolysates
The measure of solutions with a larger molecular weight than each membrane was measured as retentates, and solutions with a smaller molecular weight than each membranes was measured as permeates [7]. The moisture content of samples was measured by preheating at 80°C and drying at 105°C using oven drying method. The protein, fat, lactose, and calcium of samples were measured by International dairy federation methods.
3. Preparation of casein hydrolysates
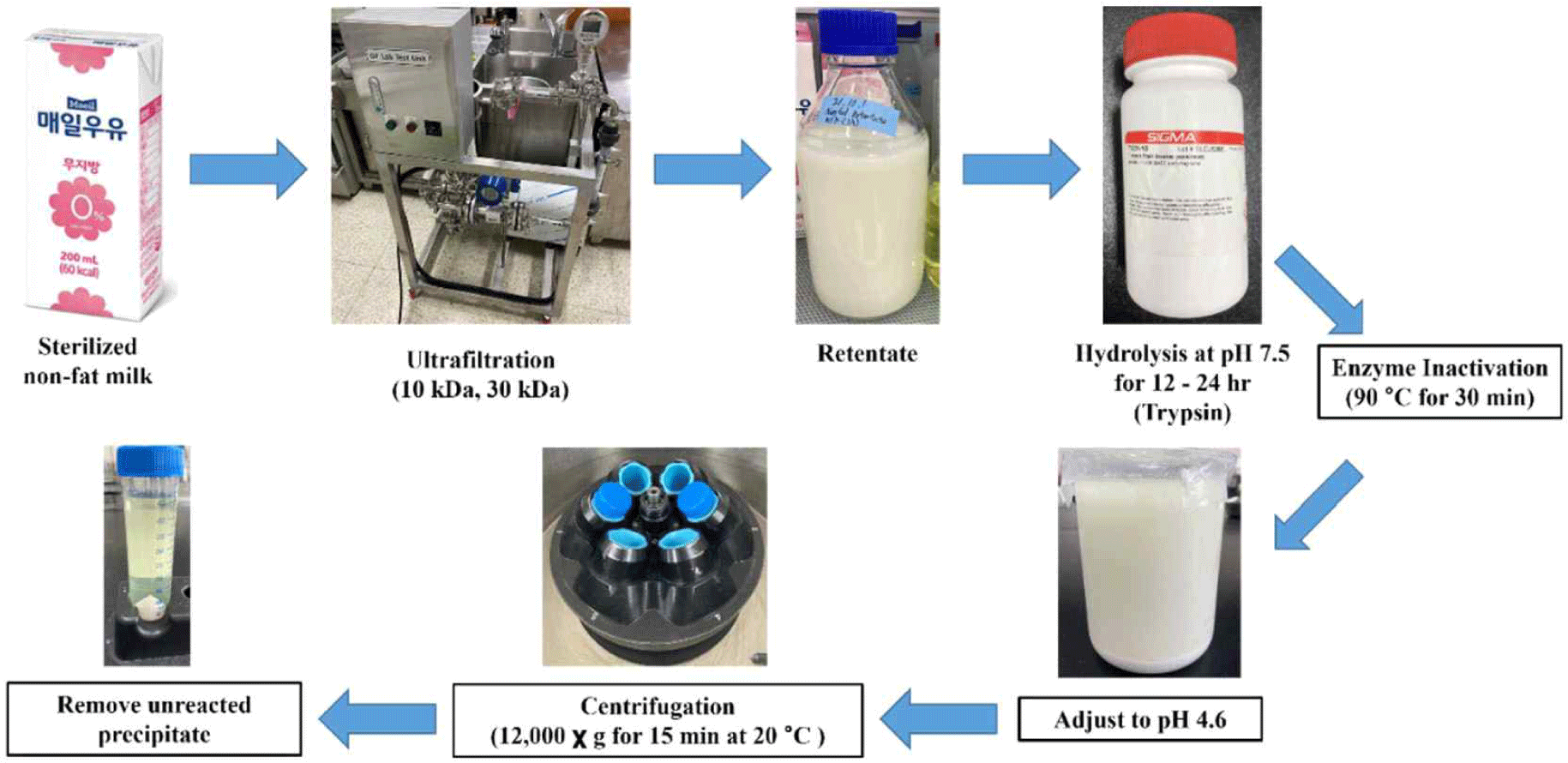
Casein hydrolysates were obtained through digestion of concentrated skim milk with trypsin. After separating non-fat sterilized milk with 10 kDa and 30 kDa membranes (i.e., 10 kDa retentate and 30 kDa retentate), 0.01% (w/w) trypsin was added and hydrolysis was performed at 37°C and pH 7.5. To inactivate the enzymes of the digested retentates, heat treatment was performed at 90°C for 30 min and pH of hydrolysates was adjusted to 4.6 to precipitate unreacted casein. The milk in which casein was precipitated was centrifuged at 12,000×g for 15 min to obtain casein hydrolysates. The hydrolysates were then either spray-dried and freeze-dried (Fig. 2).
Fig. 2.
Production of casein hydrolysates using sterilized non-fat milk by ultrafiltration lab test unit.
Download Original Figure
4. Electrophoresis (sodium dodecyl sulphate-polyacrylamide gel electrophoresis [SDS-PAGE])
Reducing SDS-PAGE was performed as SDS-PAGE kit (Mini-PROTEAN Precast Gels, Bio-Rad, USA) to find out the protein fractionation of retentates, permeates and casein hydrolysates. All gels were Coomassie-stained [8,9].
5. Statistical analysis
The Statistical Analysis System 9.4 (SAS Institute, USA) software was used to perform ANOVA. Results are presented as mean±SD of three replicates determinations. The level of significance was set at 5% level (p<0.05).
Results and Discussion
1. Composition of concentrated milk using 10 kDa and 30 kDa membranes
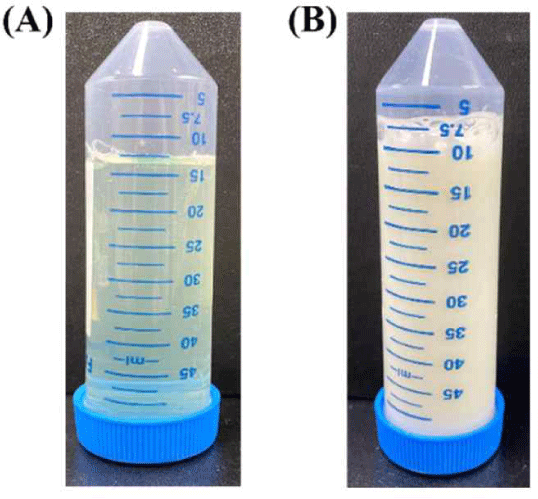
Non-fat milk was separated using 30 kDa and 10 kDa membranes of UF. Fig. 3 shows a photograph of permeates and retentates separated on a 10 kDa membranes.
The composition of retentates and permeates on 4 hr (30 kDa) and 10 hr (10 kDa) in each membrane are shown in Table 1. The total solids, protein and calcium content of retentates separated with a 30 kDa and 10 kDa cut-off were increased significantly (p<0.05) compared to those of feed. Table 2 Shows the composition of retentate and permeate on 10 kDa membrane based on VCF unit. There was a significant (p<0.05) increase in total solids and protein content of retentates when VCF was increased to 1.2 and to 1.4 (Table 3).
Table 1.
Membranes used for filtration of concentrated skim milk
| Membrane code |
10 kDa |
30 kDa |
| Molecular mass cut-off (Da) |
10,000 |
30,000 |
| Membrane diameter (I.D/O.D) |
0.9 mm/1.6 mm |
| Effective membrane area |
0.14 m2 |
| Membrane material |
Polysulfone |
| Potting material |
Urethane |
| Module case |
Polyvinyl chloride (PVC) |
| Module diameter |
Ø34 (25A) |
| Module length |
320 mm (Approx.) |
| Filtration type |
Cross-flow |
Download Excel Table
Table 2.
Composition of concentrated milk using 10 kDa and 30 kDa membranes
| Variables |
Moisture (%) |
Protein (%) |
Fat (%) |
Lactose (%) |
Calcium (mg/100 g) |
| Feed (non-fat milk) |
91.20±0.01a |
3.59±0.08c |
0.24±0.20a |
4.20±0.61a |
108.89±11.66c |
| Retentate (30 kDa) |
90.08±0.05b |
5.88±0.02b |
0.28±0.04a |
3.75±0.13a |
139.02±4.56b |
| Retentate (10 kDa) |
87.84±0.05c |
7.27±0.14a |
0.55±0.39a |
4.27±0.74a |
195.77±4.85a |
| Permeate (30 kDa) |
- |
0.42±0.20d |
0.25±0.04a |
4.62±0.05a |
21.78±4.31d |
| Permeate (10 kDa) |
- |
0.48±0.16d |
0.19±0.09a |
4.04±1.09a |
20.17±1.58d |
Download Excel Table
Table 3.
Composition of concentrated milk using 10 kDa according to VCF unit
| Variables |
Moisture (%) |
Protein (%) |
Fat (%) |
Lactose (%) |
Calcium (mg/100 g) |
| Feed (non-fat milk) |
91.20±0.01a |
3.59±0.08c |
0.24±0.20ab |
4.20±0.61b |
108.89±11.66b |
| Retentate (1.2 VCF) |
90.22±0.04b |
3.99±0.09b |
0.44±0.04a |
4.28±0.11b |
122.66±0.01ab |
| Retentate (1.4 VCF) |
89.20±0.02c |
4.66±0.03a |
0.27±0.04ab |
4.85±0.14a |
127.17±2.09a |
| Permeate (1.2 VCF) |
- |
0.24±0.00d |
0.37±0.03ab |
4.00±0.67ab |
21.65±1.91c |
| Permeate (1.4 VCF) |
- |
0.24±0.00d |
0.19±0.01b |
4.14±0.38ab |
22.53±1.27c |
Download Excel Table
2. Electrophoresis of permeates, retentates and casein hydrolysates and chemical composition of casein hydrolysates
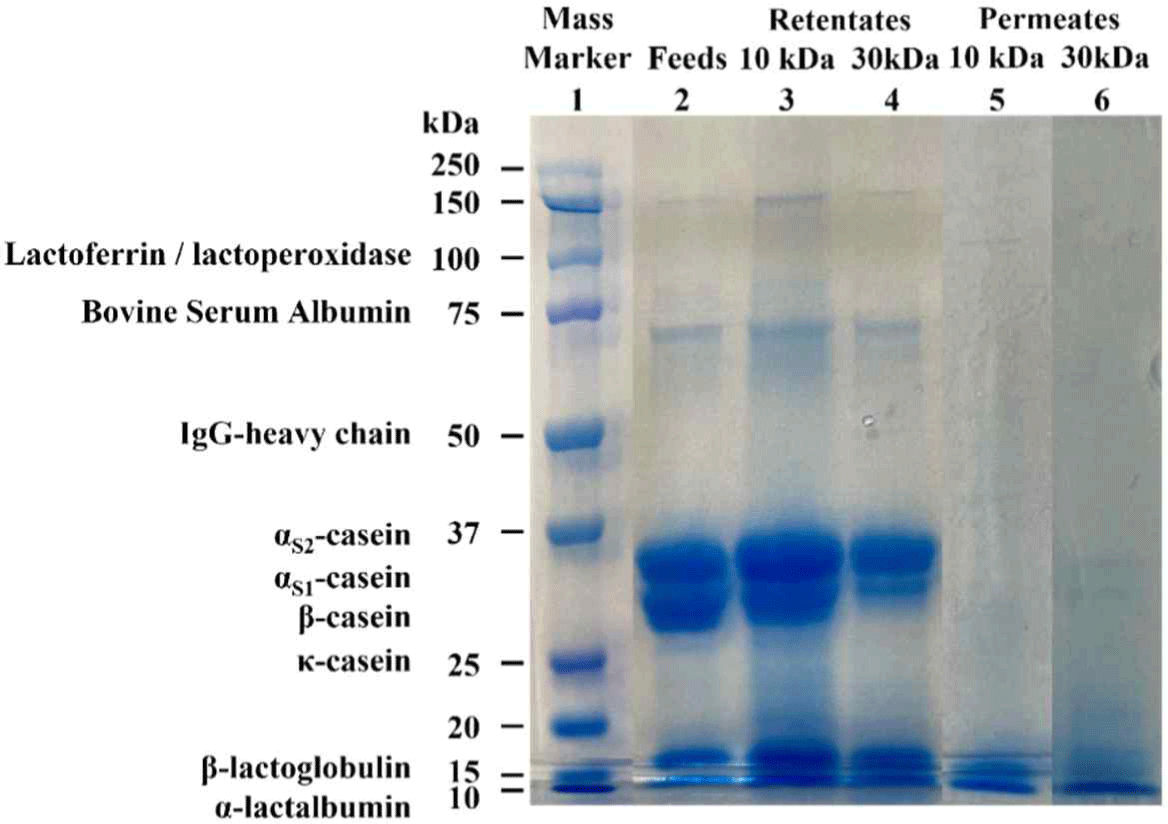
The results of SDS-PAGE of retentates and permeates using 10 kDa and 30 kDa membrane are shown in Fig. 4. In the retentate, similar protein bands with feed were observed indicating that protein in milk was concentrated after UF. The permeate protein band showed that substances with large molecular weights than membrane pores such as casein, did not penetrate. Darker band was detected in the 10 kDa retentates compared to that of the 30 kDa retentates indicating lower protein content of the feed separated from the 30 kDa membranes (Table 2).
Fig. 4.
Electrophoretic patterns under reducing conditions of retentate and permeate samples with 10 kDa and 30 kDa membrane. (1) Mass marker: ladder, (2) Feed: sterilized skim milk, (3) Retentates 10 kDa: retentates obtained by using 10 kDa membrane, (4) Retentates 30 kDa: retentates obtained by using 30 kDa membrane, (5) Permeates 10 kDa: permeates obtained by using 10 kDa membrane, (6) Permeates 30 kDa: permeates obtained by using 30 kDa membrane.
Download Original Figure
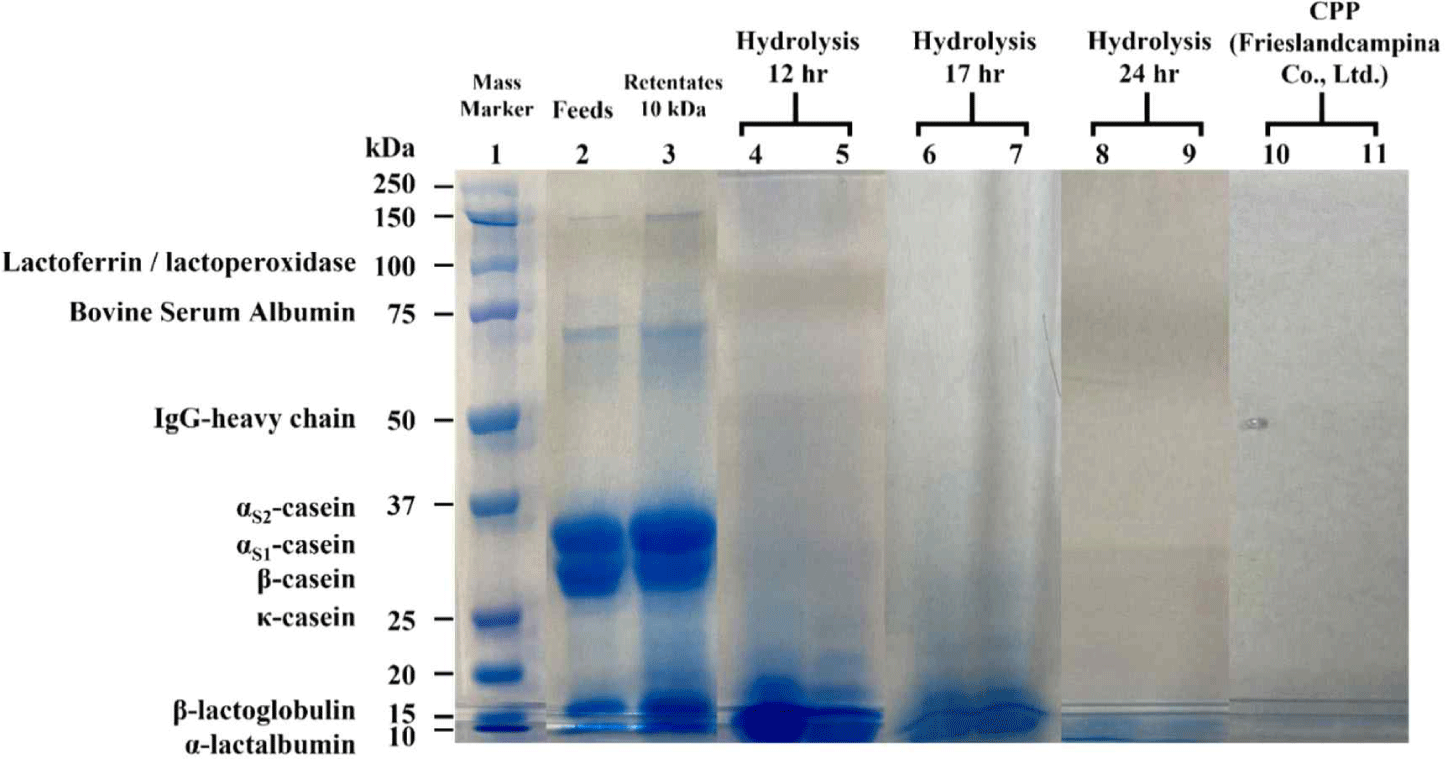
The results of SDS-PAGE of trypsinized retentates using 10 kDa and 30 kDa membrane and CPP from Frieslandcampina (Netherlands) are shown in Fig. 5. In retentate, the casein protein band was not detected after enzyme treatment. It seems that longer hydrolysis resulted in more amount of products, i.e., disappearance in casein bands after 24 hr hydrolysis. No casein bands was detected in commercial CPP as well (Fig. 5).
Fig. 5.
Electrophoretic patterns under reducing conditions of casein phosphopeptide and hydrolysis casein samples with 10 kDa membrane. Bands represent samples from two independent runs at each hydrolyzed time. (1) Mass marker: ladder, (2) Feed: sterilized skim milk, (3) Retentates 10 kDa: retentates obtained by using 10 kDa membrane, (4, 5) Hydrolysis 12 hr: hydrolyzed casein obtained by retentate using 10 kDa membrane with hydrolyzed during 12 hr, (6, 7) Hydrolysis 17 hr: hydrolyzed casein obtained by retentate using 10 kDa membrane with hydrolyzed during 17 hr, (8, 9) Hydrolysis 24 hr: hydrolyzed casein obtained by retentate using 10 kDa membrane with hydrolyzed during 24 hr (10, 11) CPP: casein phophopeptide (Frieslandcampina).
Download Original Figure
It seems that calcium in casein was effectively concentrated after UF and remained in casein hydrolysates even after enzyme treatment (Tables 2 and 4). In commercial CCP, much lower (p<0.05) Ca was detected indicating that acidification of milk was performed to produce casein hydrolysates.
Table 4.
Composition of commercial casein phosphopeptides and casein hydrolysates from UF milk
|
|
Moisture (%) |
Protein (%) |
Fat (%) |
Lactose (%) |
Calcium (mg/100 g) |
| Casein phosphopeptide (Frieslandcampina) |
6.11±0.09b |
83.64±0.10a |
0.31±0.01a |
N.D.1) |
81.83±1.08b |
| Casein hydrolysates |
12.43±0.19a |
32.03±0.03b |
0.42±0.11a |
42.09±0.24 |
1,236.03±15.63a |
Download Excel Table
Conclusion
By using ultrafiltration, milk protein was very effectively concentrated upto more than two times. During UF, the pH of milk was maintained as ∼6.7 indicating that casein micelles was not disrupted. Hydrolysis of concentrated UF milk resulted in lower molecular weight of protein compared to that of casein in milk. The Ca was also concentrated after UF and still present with a high amount in casein hydrolysates. Therefore, using UF techniques, casein hydrolysates was able to be obtained without acidification or renneting of milk and can be good sources of dairy Ca and protein for further uses as an ingredient.
Acknowledgements
This research was supported by Daegu University, 2018.
References
Fox PF. Encyclopedia of dairy sciences. 2nd ed. London, UK: Elsevier; 2011. p. 458-464.


Meisel H. Biochemical properties of regulatory peptides derived from milk proteins. Biopolymers. 1997;43:119-128.


Kelly PM. Milk protein products: membrane-based fractionation. In: Fuquay JW, editor. The encyclopedia of dairy sciences. 2nd ed. London, UK: Elsevier; 2011. p. 864-872.


Peterson RF, Nauman LW, McMeekin TL. The separation and amino acid composition of a pure phosphopeptone prepared from β-casein by the action of trypsin. J Am Chem Soc. 1958;80:95-99.


Ono T, Ohotawa T, Takagi Y. Complexes of casein phosphopeptide and calcium phosphate prepared from casein micelles by tryptic digestion. Biosci Biotechnol Biochem. 1994;58:1376-1380.


Lim K, Oh S, Park DJ, Imm JY. Recovery of milk mineral from concentrated skim milk ultrafiltration permeate. J Milk Sci Biotechnol. 2015;33:153-157.

Crowleya SV, Caldeoa V, McCarthyb NA, Fenelonb MA, Kelly AL, O’Mahony JA. Processing and protein-fractionation characteristics of different polymeric membranes during filtration of skim milk at refrigeration temperatures. Int Dairy J. 2015;48:23-30.