Introduction
Cronobacter spp. (Enterobacter sakazakii) are recognized as very significant from a public health point of view, because of its potential to cause serious illness in susceptible infants exposed to contaminated powdered infant formula [1,2].
In particular, in the first few months of life after giving birth, the biggest concern is infant feeding.
If the immune system of infants is underdeveloped and then powdered infant formula is used as an alternative to breastfeeding [3]. Cronobacter spp. (E. sakazakii) is generally not known to be associated with breast milk consumption [4]. Therefore, various necessary control measures were established during powdered infant formula production to eliminate the risk of Cronobacter spp. (E. sakazakii)-associated disease [5].
Currently, the genus of Cronobacter includes a total of seven species: Cronobacter condimentiidl, Cronobacterdublinensis, Cronobacter malonaticus, Cronobacter muytjensii, Cronobacter sakazakii, Cronobacter turicensis, and Cronobacter universalis [6,7]. All Cronobacter spp. (E. sakazakii) can be isolated from clinical specimens [8]. However, most infections were investigated to be caused by three strains: C. sakazakii, C. malonaticus and C. turicensis [8]. It is generally accepted that premature infants, low birth weight newborns, and infants with underlying the medical conditions are at the highest risk of exacerbating severe Cronobacter spp. (E. sakazakii) infection [2,9].
A multi-year analysis of the incidence of invasive Cronobacter spp. (E. sakazakii) infection in infants revealed that it was caused by powdered infant formula endogenously or extrinsically contaminated with Cronobacter sakazakii. In addition, various causes have also been reported [10]. In addition, the clinical symptoms of Cronobacter spp. (E. sakazakii) infection can be divided into two categories. First, it causes wounds and urinary tract infection, sepsis, vaginitis and aspiration pneumonia in adults [11]. Second, it causes necrotizing enterocolitis, sepsis, and meningitis in neonates and infants [12]. Especially, more seriously, sequelae from Cronobacter spp. (E. sakazakii) infection often include developmental delay, hydrocephalus, learning disabilities, and other neurological sequelae [2].
Therefore, not only health care standards for treatment methods and infection control procedures for Cronobacter spp. (E. sakazakii) infections must be established, but also regions (continents), seasons, climates, and genetic variations must be considered [13].
Recently, various efforts have been made to detect Cronobacter spp. (E. sakazakii) with the newly updated scientific technologies [14,15]. Generally, the process of bacterial isolation and identification requires a pre-enrichment step, so it is very important to select and use a pre-enrichment broth that is selective and specific for Cronobacter spp. (E. sakazakii).
Unfortunately, it is true that the currently used pre-enrichment broth generally has low selectivity [16]. Therefore, for this reason, a single broth has not been widely adopted for both Gram-positive and Gram-negative bacteria in various foods including powdered infant formula [16].
Therefore, the purpose of this study is to determine the best pre-enrichment broth to increase the number of Cronobacter spp. (E. sakazakii) so that it can be detected by restoring Cronobacter spp. (E. sakazakii) even if it is in various adverse conditions.
After culture by adding 10 different pre-enrichment broths, which are widely used in powdered infant formula artificially inoculated with Cronobacter spp. (E. sakazakii) at various concentrations, at a ratio of 1:9, the Ct value was obtained by Real-Time polymerase chain reaction (PCR). The ability of 10 different pre-enrichment broths was evaluated by comparing each Ct value with each other.
Materials and Methods
Cronobacter spp. (E. sakazakii) and Salmonella Enteritids (non-Cronobacter spp.) were provided by Center for Food Safety and Applied Nutrition, Food and Drug Administration, USA. The bacteria tested in this study were incubated in tryptic soy broth (TSB; Becton Dickinson, USA) at about 37°C for over 18 hr.
For obtaining 3 different cell concentrations, 10-fold serial dilutions were progressed in phosphate-buffered saline (pH 7.2). Viable cell concentrations of Cronobacter spp. (E. sakazakii) were determined by direct plating to DFI (Chromogenic Cronobacter spp. [E. sakazakii] agar, CM1055, Oxoid, USA) following incubation at 37°C for 24 hr. The powdered infant formula were bought from a retail/wholesale stores in College Park, MD, USA. Twenty-five gram (25 g) of the powdered infant formula was artificially inoculated at 3 different target levels such as high (7.2 CFU/g), medium (1.82 CFU/g) and low (0.82 CFU/g), respectively. Then, the inoculated test part was added to each of 10 different pre-enrichment cultures, and the Ct values were compared using Real-Time PCR (Fig. 1).
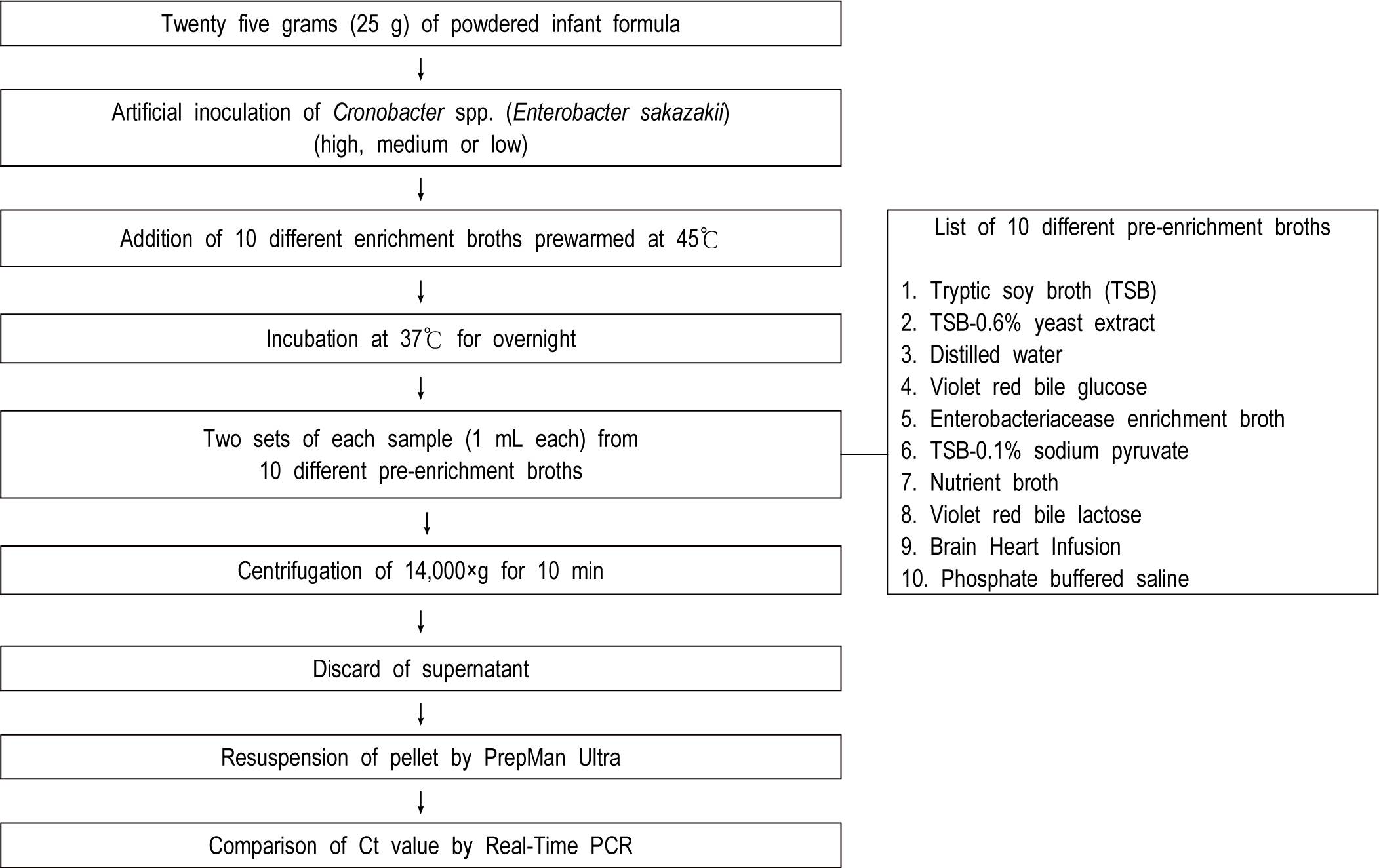
The Real-Time PCR for detecting Cronobacter spp. (E. sakazakii) was first introduced by Seo and Brackett [17]. The detection target using Real-Time PCR was the dnaG gene in the macromolecular synthesis (MMS) operon of Cronobacter spp. (E. sakazakii; Table 1).
| Type | Location within the MMS gene | Temperature of denaturation (°C) | Sequence (5’ to 3’) | Reference | |
|---|---|---|---|---|---|
| Probe | MMS | 225-258 | 70 | 6-carboxyfluorescein (the reporter dye) – agagtagtagttgtagaggccgtgcttccgaaag-6-carboxytetramethylrhodamine (the quencher dye) | [17] |
| Primer | Forward | 201-222 | 60 | gggatattgtcccctgaaacag | |
| Backward | 278-260 | 59 | cgagaataagccgcgcatt | ||
Two sets of each sample (1 mL) from 10 different pre-enrichment broths were centrifuged at 14,000×g for about 10 min (Fig. 1). In order to secure the validity of this study, true positive (Cronobacter spp. [E. sakazakii] with over 108 CFU/mL), true negative (Salmonella Enteritidis with over 108 CFU/mL), and control (deionized water) were also investigated. The cell’s pellets were resuspended in PreMan Ultra (Applied Biosystems, USA) and also located at the boiling water (100°C) for about 10 min. Hence, samples were cooled during about 2 min at 15°C to 25°C and centrifuged at 14,000×g during about 10 min repeatedly. Fig. 2 showed the cycling conditions and PCR mixture composition of the ABI Prism 7000 SDS platform for the detection of Cronobacter spp. (E. sakazakii).
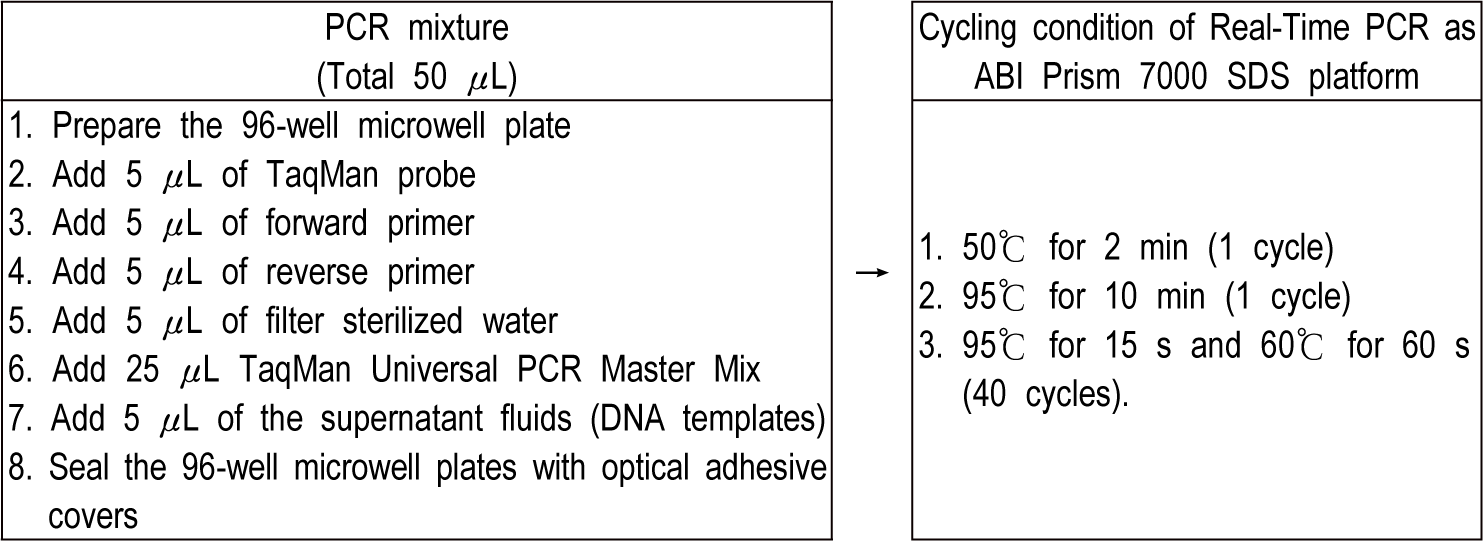
Consequently, the Ct values of samples extracted from 10 different pre-enrichment broths were compared with ABI Prism 7000 SDS, a Real-Time PCR machine. The availability of 10 different pre-enrichment broths was confirmed using the Ct value.
Results and Disscussion
Neonatal and infant meningitis, sepsis, and necrotizing enterocolitis are generally known to be associated with Cronobacter spp. (E. sakazakii) [1,2,6–17]. Therefore, accurate and rapid detection and isolation of Cronobacter spp. (E. sakazakii) from contaminated samples is very important [1,2,14,15,17]. According to the US FDA method, several steps must be taken to isolate and enumerate Cronobacter spp. (E. sakazakii) from dehydrated powdered infant formula. For example, the first is pre- enrichment, the second is subculture, the third is re-cultivation, and the fourth is yellow colony selection and striping, and so on. This process not only takes at least 6 to 7 days, but also needs to be confirmed through a Biotyping Assay such as Vitek, API 20E, and so on [2,17,18]. The pre-enrichments used here were Enterobacteriaceae enrichment (EE) broth [18]. Furthermore, According to BAM Chapter 29 (Cronobacter), revised in April 2018, the pre-concentrate used here was buffered peptone water (BPW) [18].
Hence, this study was to determine the capability of 10 different pre-enrichments using by Real-Time PCR for promoting the FDA method. First, 25 g of powdered infant formula was artificially inoculated with Cronobacter spp. (E. sakazakii), and then 225 mL of 10 different pre-enrichment broths (prewarmed to about 45°C) was added and then gently stirred until the powdered infant formula was uniformly suspended. In addition, the reconstituted preparation samples was incubated at 37°C for more than 18 hours, and DNA was extracted after collecting 1 mL from each of 10 different pre-enrichment broths. Then, after measuring the Ct value by real-time PCR analysis, the most suitable pre-enrichment broths for Cronobacter spp. (E. sakazakii) were identified. Also, the Ct value obtained using real-time PCR generally showed a low number when the cell populations of target bacteria were high [17].
First, when the inoculum of Cronobacter spp. (E. sakazakii) was 7.2 CFU/g, the Ct value appeared in the order of 21.37 (EE broth), 21.95 (brain heart infusion [BHI]), 22.72 (TSB), 23.02 (violet red bile lactose [VRBL]), 22.31 (TSB-0.1% SP), 23.43 (distilled water [DW]), 24.34 (phosphate buffered saline [PBS]), 24.95 (nutrient broth [NB]), 25.82 (TSB-0.6% YE), and 28.27 (violet red bile glucose [VRBG]; Table 2).
PCR, polymerase chain reaction; TSB, tryptic soy broth; YE, yeast extract; DW, distilled water; VRBG, violet red bile glucose; EE, Enterobacteriaceae enrichment; SP, sodium pyruvate; NB, nutrient broth; VRBL, violet red bile lactose; BHI, brain heart infusion; PBS, phosphate buffered saline; ND, not detected.
Second, when the inoculum of Cronobacter spp. (E. sakazakii) was 1.82 CFU/g, the Ct value appeared in the order of 20.34 (EE broth), 22.16 (TSB-0.6% YE), 22.37 (BHI), 22.71 (VRBL), 22.88 (TSB), 23.01 (DW), 23.19 (NB), 23.79 (TSB-0.1% SP), 24.66 (VRBG), and 24.70 (PBS; Table 3).
PCR, polymerase chain reaction; TSB, tryptic soy broth; YE, yeast extract; DW, distilled water; VRBG, violet red bile glucose; EE, Enterobacteriaceae enrichment; SP, sodium pyruvate; NB, nutrient broth; VRBL, violet red bile lactose; BHI, brain heart infusion; PBS, phosphate buffered saline; ND, not detected.
And third, when the inoculum of Cronobacter spp. (E. sakazakii) was 0.182 CFU/g, the Ct value appeared in the order of 21.93 (VRBL), 23.07 (TSB-0.6% YE), 23.31 (DW), 23.47 (PBS), 23.70 (BHI), 24.14 (TSB-0.1% SP), 25.14 (TSB), 29.00 (VRBG), 31.55 (EE broth), and undetected (NB; Table 4).
PCR, polymerase chain reaction; TSB, tryptic soy broth; YE, yeast extract; DW, distilled water; VRBG, violet red bile glucose; EE, Enterobacteriaceae enrichment; SP, sodium pyruvate; NB, nutrient broth; VRBL, violet red bile lactose; BHI, brain heart infusion; PBS, phosphate buffered saline; ND, not detected.
Consequently, this results exhibited there was not any significant difference between 10 different pre-enrichment broths.
In a similar study to this study, 10 enrichment broths were evaluated for their ability to aid the growth in artificial inoculations of low numbers of fastidious aerobic, microaerobic and anaerobic bacteria [19]. When 10 CFU was artificially added, most of the strains investigated performed best in cooked meat broth, fastidious anaerobic broth, and thioglycolate medium USP. Therefore, the above three enrichment broths are judged to be suitable as enrichment broth in clinical studies [19].
To date, no enrichment broth can support the growth of all microorganisms [19]. Therefore, it is important to first consider the factors affecting the choice of the enrichment broth so as to isolate pathogenic bacteria [19]. For example, the sensitivity of the broth for recovery of fastidious bacteria, the types of bacteria expected to be recovered, the types of specimens to be sampled, the easily preparation and use of the enrichment broth, the purchase price of the broth, and so on [19].
Also, when four substances (0.1 g/L sodium pyruvate, 0.5 g/L ammonium iron (III) citrate, 40 mM 8-hydroxyquinoline, and 0.1 g/L sodium deoxycholate) were added to BPW, the detection of Enterobacteriaceae in samples was improved [20]. Therefore, it is considered that it will be very helpful in detecting specific food poisoning pathogens belonging to the Enterobacteriaceae (Cronobacter spp., Salmonella spp. etc.) that require a pre-enrichment step in BPW [20]. Furthermore, it is considered that the improvement of the composition of BPW can potentially improve the recovery of Gram-negative bacteria from the sample in terms of suppression of the competing gram-positive background flora [20].
According to another study, the main difference is that universal pre-enrichment broth has a higher buffering capacity than BPW [21]. For this reason, universal pre-enrichment broth is more advantageous for samples containing damaged Salmonella [21]. Also, an important component constituting universal pre-enrichment broth is sodium pyruvate, which is known to play the significant role for restoring the damaged bacteria [21].
Six enrichment broths (BPW, Lactose broth, BHI, UPB, NB, and TSB) were investigated to select the optimal enrichment broth of damaged Salmonella from the refrigeration temperature [22]. Among them, the use of BHI was selected as the most optimal medium for enrichment of Salmonelladamaged from cold damage [22].
Universal pre-concentration broth is effective when detecting bacteria with heat damage, whereas Listeria enrichment broth is effective when the target bacteria are intact and a high background of the bacterial flora is expected [23].
Regardless of the food matrix and the number of background bacteria, no significant difference was observed in the detection of Cronobacter spp. (E. sakazakii) in mEE broth supplemented with sodium citrate compared to EE broth by conventional culture methods [24]. On the other hand, as a result of Real-Time PCR analysis, there was a statistically significant difference between mEE broth and EE broth [24].
According to a study comparing three commonly used Cronobacter spp. (E. sakazakii) enrichment broths (EE broth, Cronobacter spp. [E. sakazakii] selective broth, and modified lauryl sulfate broth) with a newly developed Enterbacter sakazakii enrichment broth, 177 strains (100%) grew in Cronobacter spp. (E. sakazakii) enrichment broth but between 2% and 6% of strains did not grow in EE broth, Cronobacter spp. (E. sakazakii) selective broth, or modified lauryl sulfate broth [25]. Nonetheless, it is not selective enough to qualify as a practically replaceable enrichment broth. Therefore, continuous development is required as an enrichment broth that is selective and effective against Cronobacter spp. (E. sakazakii) [25].
Conclusion
In conclusion, this study investigated the ability to detect Cronobacter spp. (E. sakazakii) using 10 different pre-enrichment broths. In other words, after adding 10 different pre-enrichment broths to the powered infant formula artificially inoculated at 3 different concentrations, the Ct value was analyzed by Real-Time PCR. This study did not show any statistically significant difference (p<0.05). Namely, all 10 different pre-enrichment broths tested in this study showed the same detection ability.
However, additional research must be conducted to rapidly and accurately detect Cronobacter spp. (E. sakazakii) in various samples including powdered infant formula. Furthermore, future studies of 10 different pre-enrichment broths that could improve the recovery rate of Cronobacter spp. (E. sakazakii) from acid-, antibiotic-, cold-, and heat-damage and also the limit of detection at very low Cronobacter spp. (E. sakazakii) concentrations would have to proceed.






