Introduction
The fermented milk manufactured using Tibetan Mushroom Grain has long been consumed by Tibetan monks in temples [1–4]. It is known that it has been used to treat diseases in the private sector as many usefulness have been known so far [1–5]. It is a type of fermented milk consisting of grains in which lactic acid bacteria and yeast coexist simultaneously [1,2,4]. In this respect, it is different from traditional yogurt fermented solely by lactic acid bacteria [3,4]. Especially, it was named the Tibetan mushroom because its shape was similar to that of mushrooms [1–5].
The characteristic of the Tibetan mushroom grain is its non-standardized form and has gelatin-like elasticity [4,5]. It is also formed by symbiosis of yeast and lactic acid bacteria, and could produce lactic acid fermentation and alcoholic fermentation in milk [3–5]. For this reason, the Tibetan mushroom was misclassified as Kombucha or Kefir, known as the naturally produced microbial symbiotic [2,4–7]. Unlike Kombucha and Kefir, creating a thin film similar to leaves around the grain is a unique feature of the Tibetan mushroom [2,3,5]. So far, there has been very little research on the Tibetan mushroom, but there has been a lot of research on Kefir, known to be similar to the Tibetan mushroom [1,8]. Fermented milk is manufactured using Kefir grain, which is known to have bacteria on the outside and yeast on the inside [4,5]. The characteristic of Kefir grain is a combination of lactic acid bacteria and yeast, a yellow-white popcorn-shaped infinitive, and it absorbs moisture from milk to increase its volume [9,10]. Commonly known, exopolysaccharide produces a narrow membrane form around the cell wall, or exopolysaccharide is a polysaccharide (primary or secondary metabolite) accumulated during fermentation as a viscous substance outside the cell wall [11,12]. Unlike polysaccharide of cell-wall or intracellular polysaccharide, exopolysaccharide produced by the most microbial polysaccharide is known to have high industrial availability and potential because it is very simple to recover from cultures and has relatively low refining costs [4,13].
Exopolysaccharide, produced by various lactic acid bacteria, has many possibilities as an edible polysaccharide [14]. For example, the biggest advantage of exopolysaccharide is that it is not only used to increase the viscosity of various dairy products and foods, but also can be used for various purposes such as emulsifiers, stabilizers, gelation and moisture-binding materials [4,5,14]. It has also been reported that microorganisms which produce exopolysaccharide are resistant to dehydration environments, bacteriophage (or macrophages), protozoa, antibiotics and toxins [15,16]. For this reason, there is a growing interest in lactic acid bacteria which produces exopolysaccharide.
Therefore, the objectives of this study are as follows. This study was isolated the exopolysaccharide produced by the Tibetan mushroom grain and various complex microorganisms presented in the culture, and then the inhibitory effect is confirmed by adding exopolysaccharide to major foodborne pathogenic bacteria (Staphylococcus aureus 305, Listeria monocytogenes ATCC 19114, Escherichia coli O157:H7 ATCC 42894, and Escherichia coli O55). Ultimately, it was conducted to explore the possibility of infection prevention of exopolysaccharide against various diseases caused by foodborne pathogenic bacteria.
Materials and Methods
In this study, pasteurized market milk was provided by Konkuk Milk The Tibetan mushroom grain used in this study were collected and used as a starter for manufacturing fermented milk. For ensuring sufficient activity, it was successively cultured three times a week. The method is described in detail as follows: In a sterile container, 180 g of pasteurized market milk was added, and 20 g of Tibetan mushroom grain was sterilely inoculated to prepare the samples by incubating it in a 23°C incubator (Sanyo: MIR-253, Japan) for 24 hours.
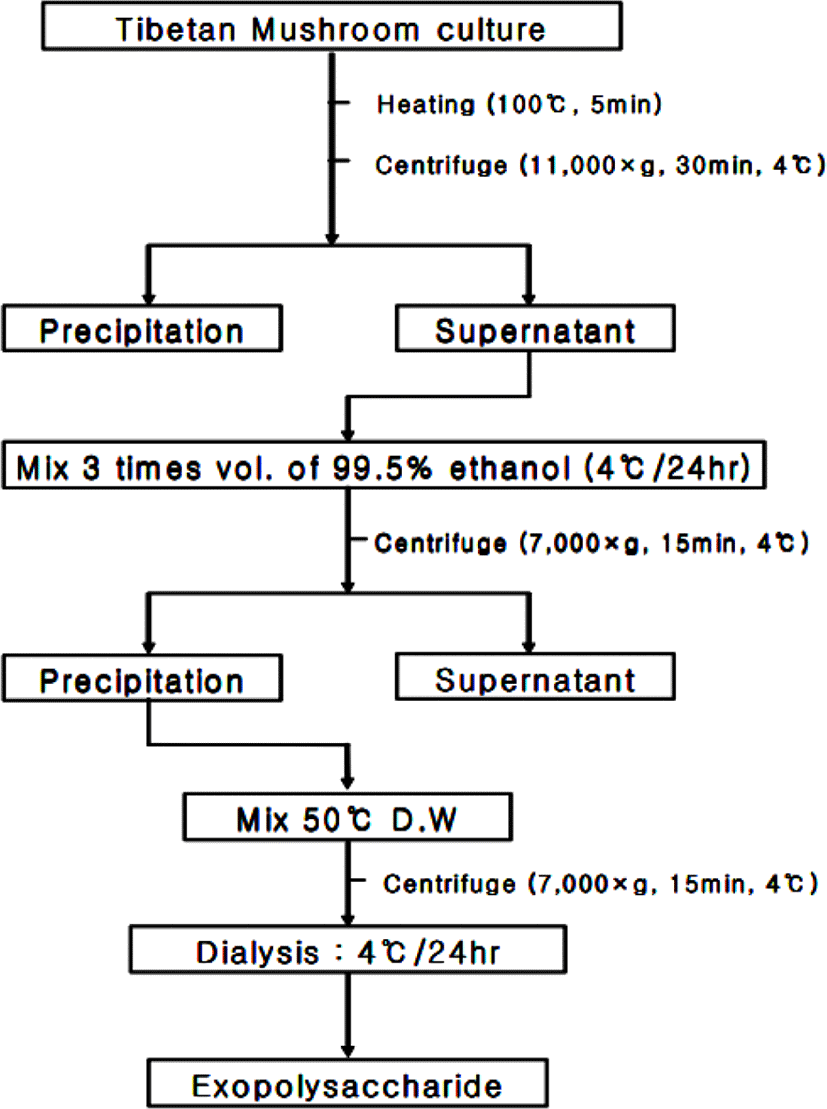
The methods of Zakaria et al. [17] and Bae & Huh [18] were applied to isolate exopolysaccharide. Briefly, the Tibetan mushroom culture was heated for five minutes to deactivate the enzyme, centrifuged (11,000×g) for 30 min at 4°C, and then the precipitates was removed. And three times the amount of the supernatant solution is added with cold ethanol (95%) to precipitate the polysaccharide at 4°C for 24 hours, and then wash it twice with the same alcohol. Precipitation was recovered through centrifugation (7,000×g) for 15 min at 4°C. The precipitation was dispersed in distilled water at 50°C, and then centrifuged again under the same conditions. Crude exopolysaccharide was subjected to 24-hour dialysis using Spectra/ProTM 2 RC dialysis membrane tubing (12,000 to 14,000 Dalton Molecular Weight Cut Off), and the obtained exopolysaccharide was frozen and dried (Fig. 1).
In this study, the strains used for testing were Gram-positive foodborne pathogenic bacteria such as Staphylococcus aureus 305, Listeria monocytogenes ATCC 19114, and Gram-negative foodborne pathogenic bacteria such as Escherichia coli O157:H7 ATCC 42894, Escherichia coli O55. The tested strains stored at –72°C were transferred to blood agar (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) plate and incubated at 37°C for 24 hours so as to regain vitality before being used in this study.
Antibacterial effects of Tibetan mushroom-derived exopolysaccharide was analyzed using agar well diffusion assay in this study [19,20]. One hundred microliter (100 μL) cultures of Gram-positive foodborne pathogenic bacteria such as Staphylococcus aureus 305 and Listeria monocytogenes ATCC 19114 and Gram-negative foodborne pathogenic bacteria such as Escherichia coli O157:H7 ATCC 42894 and Escherichia coli O55 were homogenously applied to the surface of Muller-Hinton agar (MHA, Difco, USA) plate with sterile spreader, and well was made on the surface of agar using a sterile cork-borer (5 mm diameter). Two hundred seventy microliter (270±10 μL) of exopolysaccharide (35 μg/μL) was added to this well, left at room temperature for 30 minutes to diffuse exopolysaccharide into the agar, and incubated for 22±2 hours in a 37°C incubator. All experiments were conducted three times, and the size of the clear zone (mm) that hindered the growth of microorganisms around agar well was measured in caliper to calculate the size of the antibacterial activity.
Results and Discussion
In this study, the recovery rate of exopolysaccharide in the Tibetan mushroom culture was 620 mg/L (data not shown). This result showed a higher average recovery rate of 143–350 mg/L of exopolysaccharide separated from Streptococcus thermophilus [21,22]. In other words, the average recovery rate of exopolysaccharide, isolated from lactic acid bacteria, varied widely depending on the type of lactic acid bacteria used and the incubation conditions [4,21,22].
As far as it is known, the fermented milk was manufactured by inoculating the Tibetan mushroom grains like Kefir. The effectiveness of Tibetan mushroom grains was known to prevent constipation and diarrhea, suppress harmful bacteria in the intestines, strengthen immunity, control cholesterol, and so on [1,2,4,5].
Kwon [5] published a study on the characteristic properties of fermented milk by Tibetan mushroom. The contents were as follows. The daily increase of weight was examined by inoculating pasteurized market milk with Tibetan mushroom for 10 days, and the average daily increase was 4.09 g [5]. When incubated for 48 hours, the pH decreased from 5.85 to 4.01 and the titratable acidity (TA) increased from 0.24% to 0.85% [5]. In addition, after inoculating the Tibetan mushroom with pasteurized market milk, alcohol content was gradually produced after 42 hours, increasing rapidly to 2.08% in 48 hours [5]. This was attributed to fewer lactose-fermented yeast in the composition of the yeast that forms the Tibetan mushroom [5]. The alcohol content of Tibetan mushrooms was somewhat higher than that of Marshall and Cole [23], but was approximately the same as that of Kefir, which was incubated for 48 hours [23,24]. The amino acid composition of the Tibetan mushroom grain was 14.5%–10% in order of glutamic acid (E), aspartic acid (D), alanine (A), and lysine (K), respectively [5]. And amino acid composition in fermented oils incubated 24 hours after inoculating pasteurized market milk was distributed from 17.1% to 8.3% of glutamic acid (E), leucine (L), proline (P), and aspartic acid (D), respectively [5]. However, tryptophan (W) was mostly reduced during incubation [5]. Although palmitic acid and oleic acid were distributed more than 30% of the total fatty acid composition of the Tibetan mushroom grains. However, in the case of Tibetan mushroom culture, the palmitic acid was 32.4%, followed by oleic acid, stearic acid, myristic acid, and lauric acid, respectively [5]. Finally, the carbohydrate composition of the Tibetan mushroom culture was the most common in monosaccharides containing most mannitol, followed by glucose and galactose at the same rate [5].
Therefore, systematic research is needed on how certain components of the Tibetan mushroom help improve the body’s health and prevent disease.
Generally, agar diffusion methods were widely used to measure the effects of antibacterial activity [20]. It consisted of two methods: paper disk and agar well [20]. It was known that components with the effects of antibacterial activity were diffused into agars, and the clear zone size was determined differently by their ability to inhibit [20]. However, the disk diffusion method had a disadvantage that it was not easy to accurately measure the effects of antibacterial activity of the added components because they were volatile or condensed inside the disk and cannot spread into the agar [20]. Therefore, in this study, the agar well diffusion assay method was used.
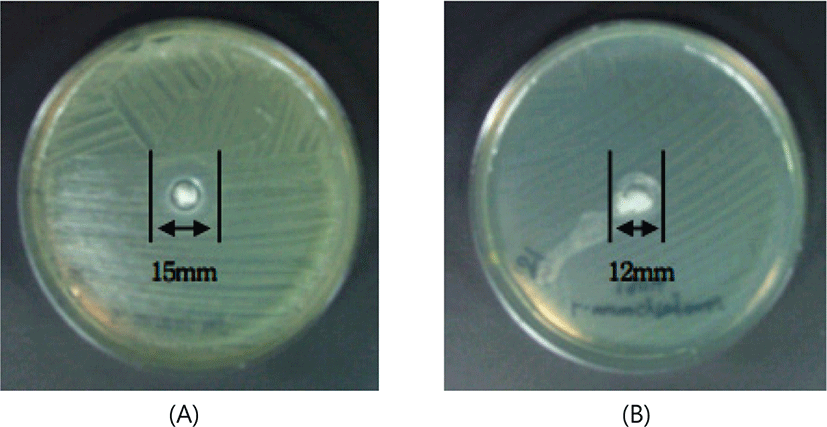
The effects of antibacterial activity of exopolysaccharide on Staphylococcus aureus 305, Listeria monocytogenes ATCC 19114, Escherichia coli O157:H7 ATCC 42894 and Escherichia coli O55 are shown in Table 1. As a result, the Gram-positive foodborne pathogenic bacteria such as Staphylococcus aureus 305 had a clear zone size of 15 mm and Listeria monocytogenes ATCC 19114 had a clear zone size of 12 mm (Fig. 2). However, the Gram-negative foodborne pathogenic bacteria such as Escherichia coli O55 and Escherichia coli O157:H7 ATCC 42894 did not form a clear zone at all (Table 1).
According to a similar other study, Jang et al. [20] compared clear zone sizes using the disk method and agar well method to measure the effect of antibacterial activity against various pathogenic food-poisoning bacteria in essential oil of Zanthoxylum schinifolium. The results reported that the agar well method was more appropriate [20].
Therefore, the results of the effects of antibacterial activity of exopolysaccharide isolated from the Tibetan mushroom in this study against various foodborne pathogenic bacteria were considered to be very significant.
So far, however, there has been a lack of overall research into the Tibetan mushroom. Therefore, it is necessary to infer the effect on the Tibetan mushroom by looking at the effects of Kefir’s antibacterial activity. For example, ingestion of Kefir could reduce the number of intestinal bacteria and Clostridia in the mouse’s colon mucosa [25], 5% kefir grain could inhibit the growth of Bacillus cereus [26], and a kefiran concentration of 300 to 1,000 mg/L could protect Caco-2 cells from Bacillus cereus [27].
Also, it had been found that the effect of Kefir’s antibacterial activity on food-poisoning pathogens were attributed to acetaldehyde, bacteria, carbon dioxide, hydrogenperoxide, organic acids [28]. Ulusoy et al. [29] reported that Kefir had the antibacterial activity against Gram-positive bacteria (Staphylococcus aureus, Bacillus cereus, and Listeria monocytogenes) and Gram-negative bacteria (Salmonella Enteritidis, and Escherichia coli). And Lactobacillus spp., isolated from Kefir, was known to inhibit the growth of Salmonella Typhimurim [29,30].
After cultivating brown rice on the mycelium of Phelinus linteus, the effects of antibacterial activity was investigated using powders extracted from Phelinus linteus mushroom rice in hot water [31]. Song et al. [31] reported that the size of clear zone of Listeria monocytogenes was observed to be 20 mm, that of Staphylococcus aureus was 18 mm, and that of Escherichia coli O55 was observed to be 13 mm, but no clear zone was indicated in Escherichia coli O157:H7 [31].
In addition, Kwon [5] examined the number of lactic acid bacteria and yeast after incubating the Tibetan mushroom in pasteurized market milk. The number of lactic acid bacteria was 1.8×108 CFU/mL at 24 hours and was 3.5×108 CFU/mL at 48 hours, and that of yeast was 1.17×107 CFU/mL at 24 hours and was 1.33×107 CFU/mL at 48 hours, respectively [5].
There is very little research on the Tibetan mushroom, but looking at the various lactic acid bacteria presented in Kefir grain, known to be similar to the Tibetan mushroom, is thought to be helpful in researching the Tibetan mushroom. The various lactic acid bacteria and yeast bacteria presented in Kefir grain have been identified as follows. There were Leuconostoc spp. (Leuconostoc dextranicum, Leuconostoc kefir, and Leuconostoc mesenteroides), Lactococcus spp. (Lactococcus cremoris, Lactococcus diacetylactis, Lactococcus durans, Lactococcus filant, Lactococcus lactis, and Lactococcus thermophiles), and Lactobacillus spp. (Lactobacillus acidophilus, Lactobacillus brevis, Lactobacillus bulgaricus, Lactobacillus caucasicus, Lactobacillus cellobiosus, Lactobacillus helveticus subsp. jogurtii, Lactobacillus kefiranofaciens, Lactobacillus lactis, and Lactobacillus plantarum) [4,32,33]. And there are two main categories of yeast in Kefir. The first was the lactose-dissolving yeasts, such as Kluyveromyces fragilis, Kluyveromyces lactis, Kluyveromyces marxianus, Saccharomyces kefir, and Tolura kefir, and the second was Saccharomyces calsbergensis and Saccharomyces cerevisiae, which are yeast bacteria that cannot break down lactose [10,32–34].
Additionally, Song et al. [35] were isolated and identified lactic acid bacteria (Lactobacillus acidophilus, Lactobacillus brevis and Lactobacillus fermentum) and yeast (Candida keffyr, Cryptococcus albidus and Pichiaohmeri) from Kefir culture and grain crushing particles. Xie et al. [1] isolated Kluyveromyces marxianus M3 from the Tibetan mushroom and after 28 days of oral administration to mice, the results were as follows. Total cholesterol in serum and liver, triglycerides, low-density lipoprotein cholesterol, and atherogenic index had been significantly reduced to help treat hypercholesterol and prevent hyperlipidemia [1].
However, in this study, the exopolysaccharide of the Tibetan mushroom showed the effects of antibacterial activity only in Gram-positive bacteria (Table 1).
Therefore, further research is needed on how exopolysaccharide in the Tibetan mushroom works against Gram-positive foodborne pathogenic bacteria. Also studies should be conducted on Gram-negative foodborne pathogenic bacteria that can have the effects of antibacterial activity if any process is added. In addition, research to identify the composition and characteristics of the Tibetan mushroom grain composed of lactic acid bacteria and yeast is urgently needed.
Furthermore, Song et al. [35] reported that exopolysaccharide isolated from Kefir exhibits inhibitory effects against pig rotavirus, cow rotavirus, and human rotavirus, simultaneously. Therefore, studies on whether exopolysaccharide isolated from lactic acid bacteria from Tibetan mushroom could inhibit various viruses are also urgently needed.
In conclusion, the Tibetan mushroom has the advantage of making fermented milk easily available to anyone. However, it is also true that there are still more unknowns, although research is being done on the various effects of the Tibetan mushroom than before. For this reason, continuous research should be conducted on the various physiological activation mechanisms of the Tibetan mushroom, and also various commercialization technologies should be actively developed so that the Tibetan mushroom would contribute to improving human health.






