Article
Functional Properties of Yogurt Containing Specific Peptides derived from Whey Proteins
Ji-Young Won1, Hong-Soek Kim2, Jin-Ah Jang1, Cheol-Hyun Kim1,*
Author Information & Copyright ▼
1Dept. of Animal Resources & Science, Dankook University, Cheonan, Korea
2Youdam Co.,Cheonan, Korea
*Corresponding Author : Cheol-Hyun Kim, Dept. Animal Resources & Science, Dankook University, Cheonan, Korea,Tel : +82-41-529-6264, Fax : +82-41-529-6264, E-mail :
hichkim@dankook.ac.kr
ⓒ Copyright 2017, Korean Society of Milk Science and Biotechnology. This is an Open-Access article distributed under the terms of the
Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits
unrestricted non-commercial use, distribution, and reproduction in any
medium, provided the original work is properly cited.
Received: Dec 06, 2017 ; Revised: Dec 15, 2017 ; Accepted: Dec 20, 2017
Published Online: Dec 31, 2017
Abstract
The purpose of this study was to investigate the acid tolerance, bile acid tolerance, and fermentation activity of lactic acid bacteria isolated from Kimchi in the presence of hydrolysates of whey protein concentrate. Kimchi isolates DK109, DK119, DK121, DK128, DK211, DK212, and DK215, which were identified as Lactobacillus sp., and L. casei DK128 showed the highest acid and bile acid tolerance. To produce whey hydrolysates, enzymes were added to a 10% (w/v) whey protein concentrate (WPC) solution at 1:50 (w/v, protein). The viabilities of the DK strains were determined in the presence of low pH and bile salts. Then, yogurt was produced via fermentation with L. casei DK128, an isolate from Kimchi, in the presence of the following additives: CPP, WPC, and WPC hydrolysates (WPCH) generated by alcalase (A) or neutrase (N). The produced yogurts were subjected to various analyses, including viable cell counts (CFU/mL), pH, titratable activity, and sensory testing. After 8 h of fermentation, the pH and titratable activity values of all test samples were 4.2 and 0.9, respectively. The viable counts of LAB were 3.49×108, 5.72×108, 7.01×108, and 6.97×108, for the Control, CPP, A, and N samples, respectively. These results suggest that whey proteins have potential as dietary supplements in functional foods and that WPCH could be used in yogurt as a low-cost alternative to CPP.
Keywords: milk protein; whey protein hydrolysis; calcium uptake capacity; anti-inflammatory effects
Introduction
Milk whey proteins are well known for their high biological value and versatile functional properties, characteristics that allow its wide use in the food and pharmaceutical industries (Julianas et al., 2015). These compact and globular proteins are responsible for 20% of the total protein contained in milk and, unlike casein, remain soluble at pH 4.6 (Asghar et al., 2011). The main proteins present in whey protein concentrates are β-lactoglobulin (3.2 g/L, 18.3 kDa), α-lactalbumin (1.2 g/L, 14.2 –kDa), serum albumin (0.4 g/L, 66.0 kDa), immunoglobulin (0.8 g/L, 146-1030 kDa), and lactoferrin (0.2 g/L, 80 kDa), among others (Foucquler et al., 2012).
The enzymatic hydrolysis of whey proteins was known to improve of their functional properties and offers interested opportunities for food science. Enzymatic hydrolysis is favored by food manufacturers due to the availability of various of enzymes that are considered safe, natural and functionalities. Many enzymes are used in the dairy industry as food-grade enzymes and others are being researched for the production of whey proteins with various functionality and biological activity.
Lactic acid bacteria (LAB) from Kimchi is a wide range of such desired roles have been suggested for diverse strains of LAB, including immune stimulation, pathogen exclusion, production of bioactive substances, and general intestinal health (Lee et al., 2011). Therefore, LAB may be more suitable if they also display desirable effects on the host (Cha et al., 2008). This suggests a potential for Lactobacilli isolated from Kimchi to be used for yogurt production.
The aims of this research are to the effect of fortification of yogurt with specific peptides isolated from WPC hydrolysates by different protease (Alcalase 2.4 L, Neutrase 0.8 L) was investigated. This study suggests that WPC hydrolysates with good nutritional and biological properties can be effectively used in health-promoting foods as a biofunctional ingredient like a yogurt product.
Materials and Methods
1. Whey protein concentrate(WPC) and commercial enzyme
Whey protein concentrate(WPC) was used commercial whey protein concentrate-80 (protein 65.45%, Kjeldahl) was obtained from Marquez brothers (USA). And commercial enzymes such as Alcalase 2.4 L (Protease from Bacillus licheniformis, 5 U/g), Neutrase 0.8 L (Protease from Bacillus amyloliquefaciens, 0.8 U/g) was purchased from Novozymes (Denmark).
2. Preparation of WPC hydrolysates(WPCH)
WPC solution was made by the WPC dissolved in distilled water at a concentration of 10% (w/v) at 40℃. After adjustment of pH to 7.0 with 2N NaOH solution, the WPC solution were pasteurization in water bath (Hansol Tech, Korea) at 65℃ for 30 min. Then, the enzymes were added to the WPC solution at a mass ratio of 1:50 (w/v, protein) in a shaking water bath at 50℃ to 200 rpm for 120 minutes. And then put in water bath at 95℃ for 10 minutes to inactivate the enzymes and stored at 4℃ until required for analysis.
3. Selection of starter
The starter used for the experiment is isolated from Kimchi. After each samples were homogenized using homogenizer (Interscience, France), 100 μL of each dilution samples were spread onto MRS agar and incubated at 37℃ for 48 h. A singular colonies were identified by 16S rRNA gene sequencing analyzed by the GeneBank database (Macrogen, Korea).
Acid and bile tolerance were performed according to the method (Teh et al., 2009 and Dayoung et al., 2014). To assay of acid tolerance, cultures were inoculated in the MRS broth adjusted to pH 2.0 with 1N HCl, then incubated at 37℃ for 2 h. For bile tolerance, cultures were inoculated in the MRS broth with 1.0% oxgall, then incubated at 37℃ for 24 h. The survival single colonies were observed at plates incubated at 37℃ for 48 h in MRS agar.
4. Manufacture of yogurt
The yogurt was made from a 92% raw milk, 3% glucose and 5% WPCH adjusted at 200 mg/mL of protein concentrate used distilled water. The raw milk and glucose mixture was pasteurized at 95℃ for 5 minutes and then added each WPCH after that cooled the mixture to 40℃, and inoculated at 0.02% (w/v) of Lactobacillus casei DK128. The mixture was incubated at 37℃ until the desired titratable acidity of 0.8 had developed. Samples were taken to measure the titratable acidity and viable cell counts. After end of fermentation added milk flavor (K-141957, KPC, Korea) 0.015% (v/v) and Yogurt flavor (971721, Symrise Pte. Ltd, Korea) 0.005% (v/v).
5. Sensory evaluation of yogurt
Sensory evaluatoin of yogurt was evaluated by A group of 30 consumer panelists was advertised at the Dankook University (Cheonan, Korea). Samples were provided to panelists in various digit random number coded paper cups. Water was provided to panelists to rinse their mouth between takes each samples. Panelists did not to talk during the sensory test.
Scores were checked on seven-point hedonic scales (1=dislike very much, 2=dislike, 3=dislike very little, 4=neither like nor dislike, 5=like a little, 6=like, 7=like very much). A seven-point hedonic was used to rate the following parameters: sweetness, bitterness, astringent, flavour, overall taste. White paper cups with samples at 10±1℃ were provided (L. Trigueros et al., 2012).
Results
1. Selection of starter
After isolation of LAB from Kimchi, Identification of strains were using 16s rDNA sequencing. DK109, DK119 and DK121 were confirmed as L. plantarum and L. paracasei on 16S rDNA gene sequencing (99% similarity to GeneBank sequences). However, DK128, DK211, DK212 and DK215 were identified as L. casei (99% similarity to GeneBank sequences) and L. paracasei (99% similarity to GeneBank sequences), respectively (Table 1).
Table 1.
Identification of isolated strains using 16s rDNA gene sequencing
| Strains |
16s-rDNA sequence |
% ID |
| DK109 |
Lactobacillus plantarum strain LP-1 |
99.0 |
| DK119 |
Lactobacillus plantarum strain ChPR-II-str62 |
99.0 |
| DK121 |
Lactobacillus paracasei strain ChR-II-str10 |
99.0 |
| DK128 |
Lactobacillus casei strain 186 |
99.0 |
| DK211 |
Lactobacillus casei strain ML7 |
99.0 |
| DK212 |
Lactobacillus casei strain LC18 |
99.0 |
| DK215 |
Lactobacillus paracasei strain 2SA3 |
99.0 |
Download Excel Table
DK strains isolated from Kimchi were measured their viabilities to low pH and bile salts. This bile salts presence creates more stressful conditions for probiotics. Therefore, resistance to low pH and bile salts are important characteristics for probiotics (Lee et al. 2012). Tolerance of eight DK strains against low pH was examined (Table 2). All DK strains killed almost completely after exposed to pH 2.0 for 2 h at 37℃. When exposed to pH 2.0 for 2 h, survival ratios were depending on each strains. DK128 showed the highest survival ratio.
Table 2.
Acid and bile acid tolerance of strains isolated from Kimchi
| Strains |
Counting (Log CFU/mL) |
| 0 h |
2 h |
24h |
| DK109 |
7.27±0.3 |
6.11±0.2 |
7.00±0.2 |
| DK119 |
8.78±0.2 |
8.48±0.3 |
8.41±0.2 |
| DK121 |
8.61±0.2 |
8.07±0.2 |
7.61±0.3 |
| DK128 |
9.12±0.1 |
8.96±0.1 |
8.96±0.2 |
| DK211 |
9.20±0.2 |
8.83±0.2 |
7.21±0.4 |
| DK212 |
8.61±0.2 |
7.53±0.2 |
8.00±0.2 |
| DK215 |
9.11±0.3 |
8.03±0.2 |
7.94±0.3 |
Download Excel Table
DK strains were the most resistant strain against 1.0% bile salts (Table 2). Other strains showed that significant reductions in the viable cell counts in the medium. The results showed that bile salt resistance property of each strains. Since 1.0% bile salts is considered to be a critical concentration used for the selection of resistant strains (Gilliland SE et al.,1984).
2. Fermentation characteristics of yogurt
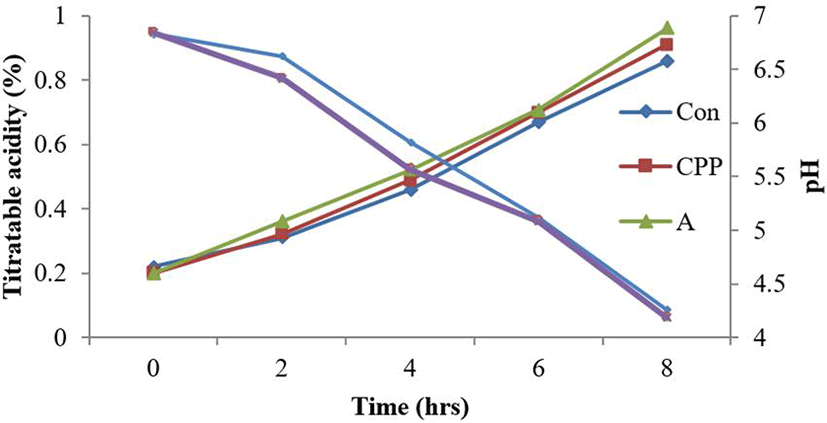
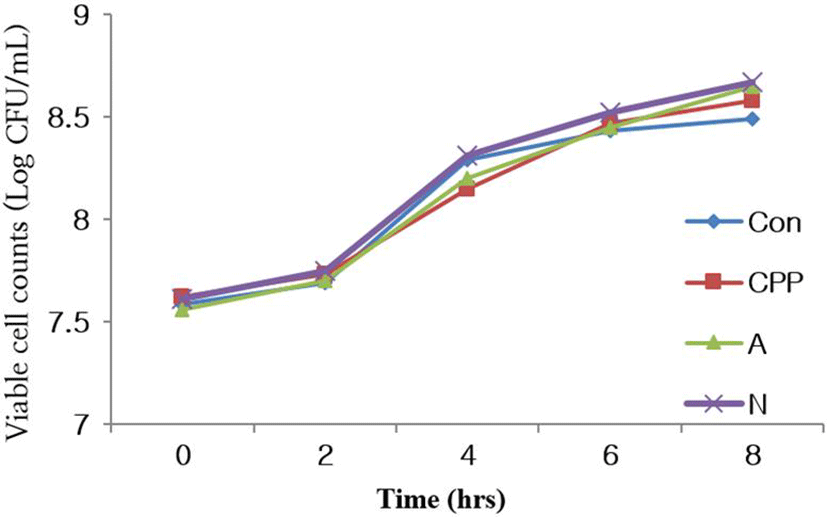
Another purpose of this study was an application of strains isolated from Kimchi to fermented dairy products as a starter culture. A starter culture should be quick and steady in lactic acid production, produce product with fine and clean lactic flavor and not produce any pigments, gas, off-flavor and bitterness in the finished products (Ghandi, 2007). Control (C), yogurt containing CPP (CPP), yogurt containing WPCH using Alcalase 2.4 L (A), and yogurt containing WPCH using Neutrase 0.8 L (N) were shown to have dramatically to decrease the pH or to increase the acidity during 8 h fermentation. The titratable acidity and pH values of all samples were 4.2 and 0.9 after 8 h fermentation (Fig. 1). C, CPP, A, and N showed growth until 8 h of fermentation and the viable cell counts (CFU/mL) of LAB during the fermentation, with values of 3.49×108, 5.72×108, 7.01× 108, and 6.97×108, respectively (Fig. 2).
Fig. 1.
Changes of pH and titratable acidity of yogurts with hydrolyzed WPC each contents during the fermentation time.
*Con : Fermentation of yogurt added nonhydrolysis WPC.
*CPP : Fermentation of yogurt added CPP.
*A : Fermentation of yogurt added hydrolysates of WPC using Alcalase 2.4 L for 2 hours.
Download Original Figure
Fig. 2.
Changes of viable cell counts of yogurts with hydrolyzed WPC each contents during the fermentation time.
*Con : Fermentation of yogurt added nonhydrolysis WPC.
*CPP : Fermentation of yogurt added CPP.
*A : Fermentation of yogurt added hydrolysates of WPC using Alcalase 2.4 L for 2 hours.
*N : Fermentation of yogurt added hydrolysates of WPC using Neutrase 0.8 L for 2 hours.
Download Original Figure
3. Sensory evaluation of yogurt
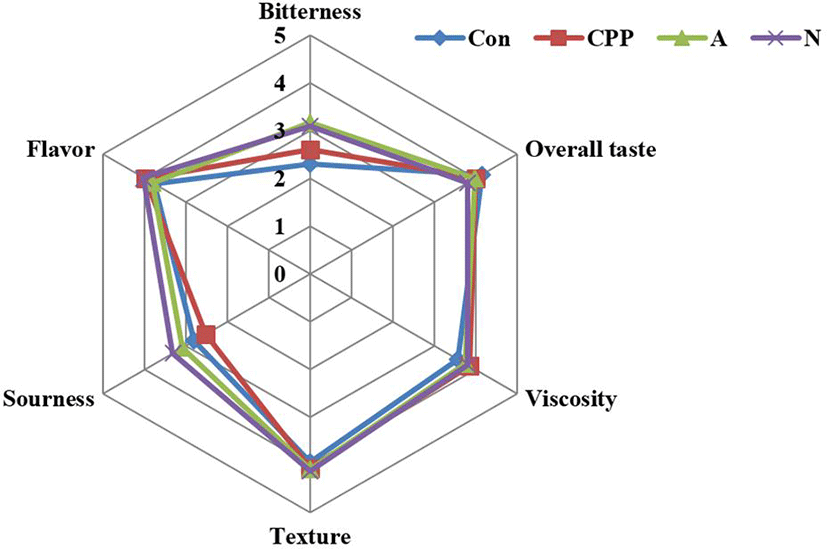
The yogurts influenced consumers’ preferences to a low extent, but it may have an important effect on the sensory quality of the evaluated products. The sensory quality of food is composed of texture, odor, appearance, and flavor attributes.
The scores recorded for Flavour, Viscosity, Sweetness, Bitterness, Astringent and Overall taste. (Fig. 3). Bitterness is negative attribute relations with food protein hydrolysates. Therefore, special attention of this experiment focused on bitterness. Bitterness of hydrolysates is more complex, and it is considered to be mainly caused by small hydrophobic peptides in the hydrolysates (R.J RitzGerald, 2006). Hydrolysates manufacturing conditions may also effect bitterness development. After hydrolysis, bitterness was increased in every samples along with hydrolysis time. This result suggest that added flavour will be decrease of bitterness of yogurts.
Fig. 3.
Spider web profile of yogurts added flavor.
*Con : WPC-80 5%(200 mg/mL, protein) in yogurt.
*CPP : casein phosphopeptide 5%(200 mg/mL, protein) in yogurt.
*A : WPC hydrolysates using Alcalase 2.4 L 5%(200 mg/mL, protein) in yogurt.
*N : WPC hydrolysates using Neutrase 0.8L 5%(200 mg/mL, protein) in yogurt.
*Added flavor 0.02%(v/v) in yogurts.
Download Original Figure
Conclusion
This study focused on fermentation yogurt of specific peptides derived from hydrolysis of whey protein concentrate using commercial enzymes. The overall sensory score of yogurts added each WPCH (5%, v/v) showed that WPCH had bitterness derived from low molecular amino acids. In this study, the results suggest that whey protein possibly has the potential to dietary supplements for functional foods and added WPCH could be alternative source for low cost material instead of CPP in yogurts.
Acknowledgements
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through High Value-Added Food Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (316062-3).
References
Amatayakul T., Sherkat, F. and Shah, N. P. 2006. Physical characteristics of set yoghurt made with altered casein to whey protein ratios and EPS-producing starter cilutures at 9 and 14% total solids. Food Hydrocolloids. 20:314-324.


Athira, S., Mann, B., Saini, P., Sharma, R., Kumar, R. and Singh, A. k. 2014. Production and characterisation of whey protein hydrolysate having antioxidant activity from cheese whey. J Sci Food Agric. Dol:10.1002.

Bassan, J. C., Goulart, A. J., Nasser, A. L., Bezerra, T. M., Garrido, S. S., Rustiguel, C. B., ... and Monti, R. 2015. Buffalo cheese whey proteins, identification of a 24 kda protein and characterization of their hydrolysates: In vitro Gastrointestinal Digestion. Journal. Pone. ID0139 550.


de Carvalho-Silva, L. B., Pacheco, M. T. B., Bertoldo, R., de Carvalho Veloso, C., Teodoro, L. C., Giusti-Paiva, A. ... and Soncini, R. 2012. Anti-inflammatory activities of enzymatic (alcalase) hydrolysate of a whey protein concentrate. African Journal of Biotechnology. 11:2993-2999.

El-Salam, M. H. A. and El-Shibiny, S. 2011. A comprehensive review on the composition and properties of buffalo milk. Dairy Sci. Technol. 91:663-699.


Gauthier, S. F. and Pouilot, Y. 2003. Functional and biological properties of peptides obtained by enzymatic hydrolysis of whey proteins. J. Dairy Sci. 86:E78-E87.


Gauthier, S. F., Pouliot, Y. and Saint-Sauveur, D. 2006. Immunomodulatory peptides obtained by the enzymatic hydrolysis of whey proteins. International Dairy Journal 16:1315-1323.


Hafeez, Z., Cakir-Kiefer, C., Roux, E., Perrin, C., Miclo, L. and Dary-Mourot, A. 2014. Strategies of producing bioactive peptides from milk proteins to functionalize fermented milk products. Food Res Int. 63:71-80.


Izquierdo, F. J., Pe-as, E., Baeza, M. L. and Gomez, R. 2008. Effects of combined microwave and enzymatic treatments on hydrolysis and immunoreactivity of dairy whey proteins. International Dairy Journal. 18:918-922.


Korhonen, H. and Pihlanto, A. 2006. Bioactive peptides: production and functionality. International Dairy Journal 16:945-960.


Madureira, A. R., Pereira, C. I., Gomes, A. M. P., Pintado, M. E. and Malcata, F. X. 2007. Bovine whey proteins- Overview on their min biological properties. Food Research International 40:1197-1211.


Nagpal, R., Behare, P., Rana, R., Kumar, A., Kumar, M., Arora, S., ... and Yadav, H. 2011. Bioactive peptides derived from milk proteins and their health beneficial potentials: an update. Food Funct. 2:18-27.



Ou, K., Liu, Y., Zhang, L., Yang, X., Huang, Z., Nout, M. R. and Liang, J. 2010. Effect of neutrase, alcalase, and papain hydrolysis of whey protein concentrates on iron uptake by Caco-2 Cells. J. Agric. Food Chem. 58:4894- 4900.



Paik, H. D., Lee, M. H., Kim, S. Y., and Toon, Y. C. 2014. Characteristics of whey protein hydrolysates from cheese whey, favors on various food application. Chem. Ind. Chem. Eng. 20:503-509.


Pintado, M. E., Pintado, A. E. and Malcata, F. X. 1999. Controlled whey protein hydrolysis using two alternative proteases. Journal of Food Engineering 42:1-13.


Silvestre, M. P. C., Morais, H. A., Silva, V. D. M. and Silva, M. R. 2013. Degree of hydrolysis and peptide profile of whey proteins using pancreatin. J. Brazilian Soc. Food Nutr. 38:278-290.


Spadaro, A. C. C., Draghetta, W., Del Lama, S. N., Camargo, A. C., and Greene, L. J. 1979. A convenient manual trinitrobenzenesulfonic acid method for monitoring amino acids and peptides in chromatographic column effluents. Analytical Niochemistry. 96:317-321.


Spellman, D., McEvoy, E., O'cuinn, G., and FitzGerald, R. J. 2003. Proteinase and exopeptidese hydrolysis of whey protein: Comparison of the TNBS, OPA and pH stat methods for quantification of degree of hydrolysis. International Dairy Journal 13:447-453.


Wroblewska, B., Karamać, M., Amarowicz, R., Szymkiewicz, A., Troszyńska, A. and Kubicka, E. 2004. Immunoreactive properties of peptide fractions of cow whey milk proteins after enzymatic hydrolysis. Int. J. Food Sci. 39: 839-850.


Yang, S. H. and Suh, J. W. 2016. Functional probiotic characterization and in vivo cholesterol-lowering activity of Lactobacillus helveticus isolated from fermented cow milk. Journal of Microbiology and Biotechnology. 26: 1675-1686. DOI: 10.4014.

Zhao, L., Huang, S., Cai, X., Hong, J., and Wang, S. 2014. A specific peptide with calcium chelating capacity isolated from whey protein hydrolysate. Journal of Functional Food 10:46-53.