Introduction
Now, the cow has become the main dairy animal associated with milk, with the term “milk” being almost synonymous with cow’s milk in most people’s minds. In fact, domestication of animals for livestock has played a key role in the development of human civilizations (Doreau and Martin-Rosset, 2002).
Milk production began 6,000 years ago or even earlier. The dairy animals of today have been developed from untamed animals which, through thousands of years, lived at different altitudes and latitudes exposed to natural and, many times, severe and extreme conditions (Rogelj, 2000). Now, the dairy industry is an essential part of agricultural policy in most countries, and these policies have resulted in the breeding of high producing stock and the development of effective and safe milk collection and delivery systems (Fox, 2008). The compositions of different types of milk are given in Table 1.
Source : Fox, 2008
According to outlook of milk consumption around the world, there has been a modest increase in the per capita consumption of milk since 1965 from 74 to 78 kg/person/year. This is predicted to rise to 90 kg/person/year by the year 2030, with rises expected in all threecategories of country (industrialized, transitional and developing) (Table 2). In terms of global importance, the increases (1965 to 1998) and the predicted increases (1998 to 2030) in East Asia and South Asia are the most significant, because of their large populations. The predicted increase in Latin America and the Caribbean is also substantial (WHO, 2003). And according to annual report of FAO, the demand for milk in developing countries is expected to increase by over 25 percent by 2025 (FAO, 2008).
Source : Bánóczy et al., 2009
Until now, milk has been part of the human diet for millennia and is valued as a natural and traditional food (Merritt et al., 2006). Milk and dairy foods are considered to be one of the main food groups important in a healthy balanced diet, and as such feature in the majority of national food-based dietary guidelines. Hence, for understanding various positive effects about milk, (1) composition of milk and dairy product, (2) several bioactive peptide derived from milk, (3) fermented milk products, (4) bone health and milk & dairy products, and (5) oral health and dietary dairy have been described in this review paper.
Cow’s Milk
Traditionally, milk is a major source of dietary energy, protein and fat (FAOSTAT, 2012). Milk is a composite liquid that provides nutrients and biological active compounds which enhance the postnatal adaptation of newborn by improving the digestive maturity, development of gut-associated lymphoid tissues and synbiotic microflora (Ebringer et al., 2008). Milk also contains antibodies which protect the young mammal against infection. A calf needs about 1,000 litres of milk for growth, and that is the quantity which the primitive cow produces for each calf. There has been an enormous change since man took the cow into his service. Selective breeding has resulted in dairy cows which yield an average of more than 6,000 litres of milk per calf, that is to say six times as much as the primitive cow. Some cows can yield 14,000 litres or more. Before a cow can start to produce milk she must have calved first. Heifers reach sexual maturity at the age of seven or eight months but are not usually bred until they are 15 – 18 months old. The period of gestation is 265 – 300 days, varying according to the breed of cow, so a heifer produces her first calf at the age of about 2 – 2.5 years (Fox, 2008).
Up to now, approximately 35 percent of dairy cows (about 70 million head) belong to the Holstein-Friesian breed. The popularity of this breed is largely because of its high average milk production (Fox, 2008) and superior ability to convert feed into protein (Buchanan, 2002). Cow’s milk accounted for 83 percent of global milk production in 2010 (FAOSTAT, 2012).
Also, milk could be classified according to its fat content, for example as whole milk, skimmed milk, semi-skimmed milk, low-fat milk and standardized milk. Also It could be classified according to the processing procedures in has undergone, such as pasteurized milk, sterilized milk, ESL (extended shelf-life) milk and UHT (ultra-high-temperature)-treated milk, among others (FAO and WHO, 2009) (Table 3).
Source : FAO and WHO, 2009; FAOSTAT, 2012
Next, among various components, cow’s milk contains approximately 3.5% protein of which 80% are casein and 20% whey proteins. Caseins have been classified as α-, β- and κ-caseins, and also whey contains alactalbumin, β-lactoglobulin and several minor proteins with different biological activities such as enzymes, mineral-binding properties and immunoglobulins (Daniel et al., 1990). The Biologically active peptides in the protein sequence could be defined as fragments that remain inactive in precursor protein sequences. However, when released by the action of proteolytic enzymes, they could interact with selected receptors and regulate the body’s physiological functions (Meisel and Bockelmann, 1999). The activity of peptides is based on their inherent amino acid composition and sequence, and the size of bioactive peptide sequences known to possess multi-functional properties could vary from two to twenty amino acid residues (Meisel and FitzGerald, 2003). Up to now, the multi-functional properties of biologically active milk peptides are increasingly acknowledged. Especially, it could show a positive impact on human’s physiology and metabolism either, directly or through enzymatic hydrolysis in vivo or in vitro (Kitts and Weiler, 2003). The protease enzymes are naturally occurring in food products (for example, milk plasmin), hydrolyze proteins and release bioactive fragments during processing or storage. Furthermore, in case of producing various fermented food products and occurring naturally in the gastrointestinal tract, many types of bacteria could be producing biologically active peptides. For example, cheese contained phospho peptides, and it would be further proteolyzed during the processing of cheese ripening. Eventually it could contribute to form the angiotensin- converting-enzyme (ACE) inhibitors (Saito et al., 2000). The various biologically active peptides derived from milk are initially found in inactive form within the sequence of the precursor molecules, however it could be released in three ways as follows; (i) the enzymatic hydrolysis with digestive enzymes such as chymotrypsin, pepsin, trypsin, and so on; (ii) the fermentation of milk with proteolytic starter cultures; (iii) the proteolysis by enzymes derived from proteolytic microorganisms (Korhonen and Pihlanto, 2003). In other words, when these bioactive peptides are liberated, they could serve to influence numerous physiological responses including cardiovascular, digestive, endocrine, immune & neurological activity, and so on (Table 4).
Owing to such various physiological versatilities mentioned above, the attention of many researchers worldwide would be focused on the research of the milk-derived bioactive peptides so as to formulate several potential new drugs with nutraceutical supplement properties, health promoting functional foods or other pharmaceutical products (Korhonen and Pihlanto, 2003; FitzGerald and Meisel, 2003). Therefore, the protein in cow’s milk is of high-quality (defined as protein that supports maximal growth), containing a good balance of all the essential amino acids, including lysine. Since many human diets are deficient in certain essential amino acids (WHO, FAO and UNU, 2007).
Fermented Milk and Dairy Products
Until now, there are more than 3 500 traditional, fermented foods worldwide (EUFIC, 1999). Fermented milk products have been reported to have a positive effect on the human digestive system (Donovan, 2006).
For several thousand years, people have been consuming fermented milks. It is an old consideration that they are health beneficial. They itself have all the milk components modified by lactic acid bacteria (LAB) fermentation (Ebringer et al., 2008). Lactic acid is produced by fermentation of lactose. It minimizes the pH, affects the casein physical properties and consequently enhance digestibility (Fig. 1). Recently, lactic acid bacteria as probiotics have been widely used in producing various fermented milk, therefore, the interest in lactic acid bacteria as probiotics has been increased. In general, probiotics could be defned as “living microorganisms, which upon ingestion in certain numbers, exert health benefits beyond inherent basic nutrition” (Tannock, 2002) but interest in this area was initiated by Metschnikov 100 years ago (Metschnikoff, 1907). Most probiotic microorganisms belong to Lactic Acid Bacteria (LAB). As for the dose of probiotics, it is important to achieve an optimal mass of probiotic in order to survive and colonize the gut, proliferating in adequate amounts, to confer a health benefit. The evidence suggests this dose should be minimally 106 to 107 CFU (colony-forming units) in each gram of product (Rautava et al., 2002).
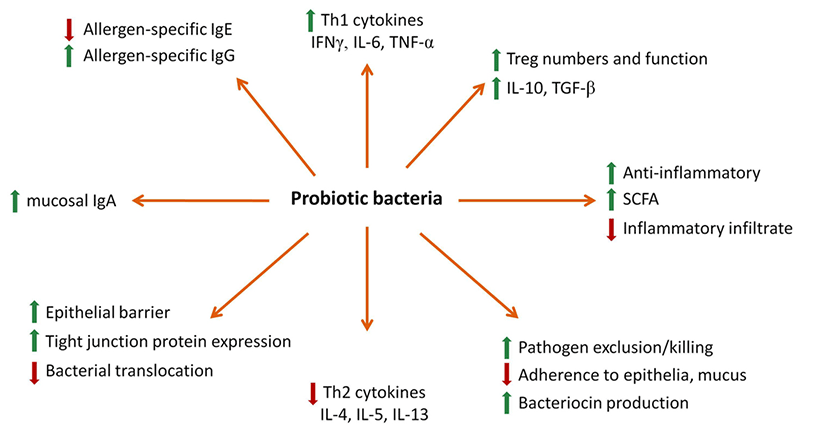
Also, it could meliorate the usage of calcium and different other minerals and suppress the development of potentially injurious bacteria. Furthermore, fermented milk could be endured by individuals having a reduced ability for lactose digestion due to its smaller lactose content (McBean, 1999), and protein degradation is due to the effect of proteolytic activity of LAB that results in some bioactive peptides and free amino acids. In fact, the bioactive peptides are a common supplement to various functional foods. Among them, milk proteins are the major resource of a variety of biologically active peptides (casokinins, casomorphins, immunopeptides, lactoferricin, lactoferrin, phosphopeptides, and so on) (Dunshea et al., 2007). Several bioactive peptides derived from milk protein are inactive inside the parent protein sequence and could be generated by enzymatic proteolysis in food processing or gastrointestinal digestion (Parvez, et al., 2006). In recent years, multiple reports have described beneficial effects from various aspects on important diseases. Anti-microbial activity, anti-thrombotic activity, blood pressure regulation, immunomodulation and mineral (or vitamin) binding are the major biological activities of such peptides (Fig. 1). Also, the fermented milks are main source of whey proteins such as immunoglobulins, lactalbumin, lactoglobulin, lactoferrin, lactoperoxidase, and so on (McIntosh et al., 1998). These proteins have exhibited a number of biological effects having various effects on the functions of digestion and anti-carcinogenic activities (McIntosh et al., 1998). During the process of fermentation, the digestibility of fat could be also improved. Even though high percentage of saturated fatty acids (FAs) is present in milk fat, it is frequently advised to avoid its use as it leads to coronary heart disease and an atherogenic profile of blood (Shah, 2007). Among the several different saturated FAs in milk, only three FAs - palmitic, myristic and lauric acids- could have the property to raise the blood cholesterol level, however, the one third of the unsaturated FAs could have the tendency to decrease the cholesterol’s level (Shortt et al., 2004). Moreover, fermented milks contain components that are also protective effect. These comprise conjugated linoleic acid (CLA), linoleic acid, calcium, probiotic bacteria or lactic acid bacteria and antioxidants (Fig. 1) (Rogelj, 2000). When the milk fat comprises of several components such as butyric acid, carotene, CLA, ether lipids sphingomyelin and vitamins A & D, it could show anti- carcinogenic effects. Several animal studies reveal that fermented milk inveterate the anti-carcinogenic action of CLA as well as its part in atherosclerosis prevention and in modulation of immune system (MacDonald, 2000).
Bone Health and Milk & Dairy Products
Recently, calcium absorption would be the most interesting content. In general, the mineral profiles in milk and bones have much in common. With the exception of small fish that are eaten whole, including the bones, few foods naturally contain as much calcium as milk (Weaver et al., 1999; Theobald, 2005). Calcium in milk has a high bioavailability, similar to calcium carbonate, which is readily absorbed (Theobald, 2005). Milk is the major source of vitamin D in the diet in countries where milk is fortified with this vitamin, for example, the United States and Canada (USDA and USDHHS, 2010). Dairy products are also a source of dietary protein. Analyses of food sources of calcium, vitamin D, protein, phosphorus and potassium in Americans reveal milk to be the number one single food contributor of most of these bone-related nutrients (Rafferty and Heaney, 2008).
On basis of the known effects of individual nutrients on calcium status, one could speculate that intake of foods such as yoghurt and milk would be advantageous (Weinsier and Krumdieck, 2000) (Table 5). Especially, potassium administration has been found to decrease urinary hydroxyproline and increase serum osteocalcin, suggesting reduced bone resorption and increased bone formation. New et al. (1997) found that food which has a high potassium contents predicted greater bone density at all 4 bone sites measured. Hence, the difference in potassium content among dairy products would be important and also it should be considered (Table 5).
Source : Weinsier and Krumdieck, 2000
The benefits to bone health of including dairy products in the diet or risks of excluding dairy products vary with the life stage. The relationship between milk & dairy products and bone mineral content and bone mineral density (BMD) was reviewed by US Department of Health and Human Services and US Department of Agriculture (USDHHS and USDA, 2005), which found that milk, foods fortified with dairy calcium and calcium supplements all had comparable effects, increasing skeletal mass in younger subjects and reducing loss of skeletal mass in older subjects. However, skeletal benefits of dairy calcium would persist longer than those derived from calcium supplements (USDHHS and USDA, 2005). Also, increased dietary calcium/dairy products, with and without vitamin D, significantly increased total body and lumbar spine bone mineral content in children with low dietary calcium in takes (450–746 mg/day) at baseline (Huncharek et al., 2008). In adolescents, controlled feeding studies with a range of calcium intakes show that dietary calcium explains 12–22 percent of the variation in skeletal calcium acquisition in girls and boys (Braun et al., 2007; Hill et al., 2008). In adolescent girls, bone mineral density (BMD) has been shown to increase by up to 10 percent when 700 mg of supplemental calcium was provided in the form of dairy products, compared with an increase of 1–5 percent when the same quantity of calcium was provided as a calcium supplement, suggesting that supplementation with dairy products has a greater effect on bone health than do calcium supplements (Kerstetter, 1995).
In a two-year randomized controlled trial (RCT), early pubertal girls receiving 1 g calcium from cheese had greater thickness of the cortical shell of the tibia than girls receiving the same amount of calcium from calcium carbonate or who received a placebo (Cheng et al., 2005). According to a seven-year intervention study, Matkovic et al. (2005) found that calcium supplementation (about 670 mg/day beyond a habitual dietary calcium intake of about 830 mg/day, giving a total calcium intake of about1 500 mg/day) affected BMD during the pubertal growth spurt but had a diminishing effect thereafter because of the catch-up phenomenon in bone mineral accretion. By young adulthood, significant effects of calcium supplementation were present at metacarpals and at the proximal forearm in subjects who had better calcium compliance and in subjects who developed larger body frames (Matkovic et al., 2005). In another study, gain in bone mineral mass in prepubertal girls was followed up three to five years after discontinuation of calcium supplementation with calcium phosphate extracted from milk incorporated in various foods, which provided on average a calcium supplement of about 850 mg/day (Bonjour et al., 2001). The authors concluded that this form of calcium phosphate taken during the prepubertal period can modify the trajectory of bone mass growth and cause a long-standing increase in bone mass accrual that lasts beyond the end of supplementation. Most RCTs in older adults use calcium and vitamin D supplements rather than dairy products (Elders et al., 1994). In one trial involving postmenopausal women that did use dairy products, adding 24 oz. of milk per day (giving a mean calcium intake during milk supplementation of 1,471 mg/ day) suppressed bone turnover and improved calcium absorption resulting in an improvement in calcium balance (Recker and Heaney, 1985).
Dairy product consumption would have particular protective effects on women taking oral contraceptives (OC). In young OC users aged 18–30 years with a habitual calcium intake of less than 800 mg/day, increasing calcium intake to 1,000-1 100 mg/day or 1,200-1 300 mg/day) using dairy products (with an emphasis on non- and low-fat milk) protected against loss of hip and spine BMD (Teegarden et al., 2005). The authors speculate that an increase in calcium absorption mediated by an increase in calcitriol (1,25-dihydroxyvitamin D) levels may explain the positive response in bone accrual noted in OC users after dairy product intervention compared with non-OC users.
Based on these studies, Weaver (2008) concluded that advantage in bone gains due to intervention generally disappeared when calcium supplements were used, but not when the intervention was dairy. Although bones may be more responsive to lifestyle choices in young people rather than later on in life, a meta-analysis showed that in premenopausal women of 18–50 years old a calcium intake of 1,000 mg/day or more was positively associated with bone mass (Welten et al., 1995). Consuming extra dairy products for three years increased calcium intake to an average of 810–1,572 mg/day, reduced vertebral BMD loss in premenopausal women (Baran et al., 1990). Furthermore, short-term treatment-related changes in bone turnover markers, especially bone formation, were strongly associated with subsequent changes in BMD (Tu et al., 2015). This could suggest that serial measurement of bone turnover shortly after initiation of kefir therapy may be helpful in assessing the ultimate therapeutic response to kefir-fermented milk (Tu et al., 2015).
Thus, dairy products represent a distinct group, presumably because of their relatively high calcium content. Calcium is considered to be important for bone health. Furthermore, Nordin et al. (1987) suggested that age-related bone loss may be more attributable to excessive calcium loss than to inadequate calcium intake. Accordingly, greater attention needs to be given to eliminating the causes of calcium loss, which in turn should lower calcium requirement (Weinsier and Krumdieck, 2000).
Oral Health and Milk & Dairy Products
In general, dental disease is the most common cause of tooth loss in developed countries (USDHHS, 2000). Tooth decay is an increasing problem in developing countries as diets change to include more sweet and processed foods (Aimutis, 2004). Since the late 1950s, milk was believed to have a protective effect on tooth enamel (Shaw et al., 1959; Jenkins and Ferguson, 1966). Milk has been suggested to have a protective effect against sugar when consumed together (Johansson and Lif Holgerson, 2011) (Table 6).
Source : Lempert et al., 2015.
The anti-cariogenic effect of dairy products has been attributed to constituents such as calcium, casein and phosphate (Aimutis, 2004). Also bioactive components in milk could reduce dental caries by changing the microbial population of dental plaque, in other words, by inhibition of adhesion of cariogenic streptococcal bacteria and establishment of less cariogenic species such as oral actinomyces (Aimutis, 2004; Johansson and Lif Holgerson, 2011). Animal studies have demonstrated reductions in dental caries when soluble calcium and phosphate salts were added to foods (van der Hoeven, 1985). Epidemiologic studies have shown that children and adults with higher concentrations of calcium and phosphate in their dental plaque had a lower incidence of dental caries (Schamschula et al., 1978). When caseinophosphopeptides from milk react with calcium and phosphate at the tooth surface they produce colloidal amorphous calcium phosphate complexes which promote remineralization of enamel in humans (Aimutis, 2004). In an in vitro study, yoghurt containing casein phosphopeptides prevented demineralization of tooth enamel and enhanced its remineralization (Ferrazzano et al., 2008). A Swedish study found that children who never ate cheese or ate it only once in the five-day period recorded had an average of 1.5 surfaces affected by caries, whereas those who ate cheese five times or more in the five-day period (namely, on average at least once a day) were caries free (Öhlund et al., 2007). A similar study in Japan suggested that high intake of yoghurt may reduce the prevalence of dental caries in children but showed no association between caries and milk or cheese consumption (Tanaka et al., 2010). The exact mechanism by which certain dairy products are anti-cariogenic is still unclear, but the current evidence suggests that consumption of these milk products can protect against dental caries (Johansson and Lif Holgerson, 2011). WHO and FAO (2003) reported that both hard cheese and milk probably decrease risk of dental caries, and that hard cheese also possibly decreases the risk of dental erosion.
Therefore, cow’s milk could be considered non-cariogenic. In vivo and in vitro demineralization and remineralization (enamel slab) experiments also indicated the low cariogenic potential of milk and also demonstrated its caries-protective role. These actions would appear to be due to (i) lactose being the least cariogenic of dietary sugars, (ii) the protective role of casein and possibly fats, and (iii) the protective role of calcium and phosphorus.
Conclusion
Milk and dairy products are healthy foods and considered nutrient-rich because they serve as good sources of calcium and vitamin D as well as protein and other essential nutrients. They provide phosphorus, potassium, magnesium, and vitamins A, B12, and riboflavin. In fact, the calcium in milk and fermented dairy products (yogurt or cheese) could have an important role of supplying calcium or vitamin D each day. And furthermore, getting the recommended three servings of dairy per day can help build bone mass, leading to improved bone health throughout the life cycle. Especially, fermented foods and beverages possess various nutritional and therapeutic properties. Lactic acid bacteria (LAB) play a major role in determining the positive health effects of fermented milks and related products. The calcium in milk is easily absorbed and used in the body, which is why milk and milk products are reliable as well as economical sources of calcium. A diet rich in protein and vitamin D contributes to bone health. Due to their high protein, vitamin D, and calcium content, dairy foods are a good choice for maintaining strong bones. A diet rich in fruit, vegetables and low-fat dairy products, with reduced saturated fat, is as effective as some medications in reducing blood pressure in people with increased blood pressure. It has also been shown to reduce risk of cardiovascular disease and type-2 diabetes. Cultured dairy products such as yogurt contain probiotics which provide a wide array of health benefits. Probiotics in the diet can enhance the good bacteria in the gut, improve health and reduce the risk of certain diseases. For more than 50 years, many studies have consistently provided evidence for the benefits of milk and dairy products on dental health. Milk and dairy products such as cheese and yogurt are beneficial to dental and oral health, and various bioactive peptides found in milk, as well as calcium, have important functions in the maintenance of dental health through enamel-protective and anti-caries effects. Hence, it needs further study the various beneficial interactions between milk & dairy product and human’s health.






