Introduction
Currently, the main cures for cancer are radiation therapy, chemotherapy, surgery; each carrying considerable risks and potential side effects. Various facets of cancer treatment are under consideration, including strategies to alleviate side effects and the integration of adjunct and complementary therapies [1]. Lactoferrin, a non-toxic globular protein that is effective on human innate immunity, has garnered attention in this context [2]. In particular, lactoferrin derived peptides such as lactoferricin B and holo lactoferrin of bovine, have been recognized for their potential as anticancer agents [3]. Numerous studies demonstrate how lactoferrin can slow cancer progression through various mechanisms [4]. Additionally, research indicates that milk lactoferrin and its peptides can inhibit cancer advancement in vitro and in vivo conditions [5]. When taken as an oral supplement at concentrations between 0.2% and 2%, lactoferrin has been shown to reduce carcinogenesis in animal models by approximately 32.5% to 42.5%. Furthermore, the application of bovine lactoferrin has shown protective effects against multiple cancer types [6].
Inflammation involving the activation of numerous immunological mediators and diverse cell types represents the most immune responses to microbial and non-microbial damage. The production of essential immunological factors, such as chemokines and cytokines, has been found to be contingent on cellular responses driven by inflammation [2]. Immunomodulators are either natural or synthetic agents that can influence the immune response by either enhancing or dampening the mediators involved in the immune cascade, which encompasses both innate and adaptive immune reactions [2].
Thus, lactoferrin serves as a bridge between adaptive and innate defense mechanisms by regulating specific cellular responses. It is acknowledged for its function in modulating antigen presentation and promoting the production of T-helper cell responses [1]. Lactoferrin is a noticeable modulator for adaptive immune functions, facilitating the conversion of immature B cells into effective antigen-presenting cells and aiding in the maturation of T cells into helper cells [7]. It is vital to the host’s initial defense against various microbial threats [5] and aids in the prevention of diseases related to inflammation [8]. Consequently, this chapter will delve into the therapeutic potential and mechanisms through which lactoferrin may inhibit cancer, as well as its prospects as a drug delivery system for the targeted administration of chemotherapeutic agents [9]. Additionally, the discussion will encompass the effect of lactoferrin as a natural modulator in inflammation-related reactions and immune responses.
Mechanisms of Lactoferrin’s Anticancer Action in Metastasis
In vitro and in vivo trials, its proved that lactoferrin, particularly bovine lactoferrin (BLF), has been extensively studied for its anticancer properties, especially concerning breast cancer, colorectal cancer, and leukaemia. Cancer cells, upon detaching from the primary tumour, acquire the ability to migrate and invade surrounding tissues, enhancing metastatic behaviour alongside resistance to apoptosis and increased proliferation. Noteworthy clinical trials have evaluated the efficacy of lactoferrin in treating and preventing metastatic cancers.
When apo-bovine lactoferrin was applied subcutaneously to lymphoma and melanoma mice, it effectively inhibited metastasis to the liver, lungs, and spleen, as well as tumour-induced angiogenesis [10]. Moreover, oral using of bovine lactoferrin and lactoferricin B in mice with aggressive colon cancer showed suppression of lung metastasis and colony formation [11]. Recent findings have indicated that a deficiency in lactoferrin resulted in increased lung metastasis due to the attraction of myeloid suppressor cells in lactoferrin knockout mice [12]. These results underscore the significant role lactoferrin plays in regulating cancer metastasis. In addition to its role in reducing cancer metastasis, lactoferrin demonstrates properties that inhibit cell migration and invasiveness. Research has shown that lactoferrin can successfully obstruct cell invasion and migration across a variety of cancers, although the main molecular mechanisms of these effects remain elusive. One critical process in cancer metastasis is transition of epithelial-to-mesenchymal (EMT), where cells develop invasive characteristics, allowing them to infiltrate blood vessels and surrounding tissues, thus enhancing metastasis. Recent studies indicated that bovine lactoferrin can reverse the EMT process in both oral cancer cells [13] and glioblastoma [14]. In cancer cells, various proteins and transcription factors, such as snail, twist, STAT3, and vimentin, are often overexpressed, leading to a suppression of cadherins—essential molecules for cell adhesion—which is associated with increased cancer aggression. Bovine lactoferrin has effectively mitigated invasiveness in the HOC313 cell line and oral cancer cells by reversing EMT [13]. Mechanistic analysis demonstrated that bovine lactoferrin binds to the LRP1 receptor, resulting in the inhibition of twist via ERK1/2 dephosphorylation. When administered orally in xenograft models, bovine lactoferrin was found to elevate cadherin levels and decrease cancer cell infiltration.
Apart from its primary mechanism, lactoferrin’s iron-binding capabilities also play a role in its anticancer effects. Given its efficacy against cancer metastasis, lactoferrin is being explored as a potential chemotherapeutic agent. Thus, further investigations are necessary to assess its applicability in treating various cancers.
Induction of Apoptosis
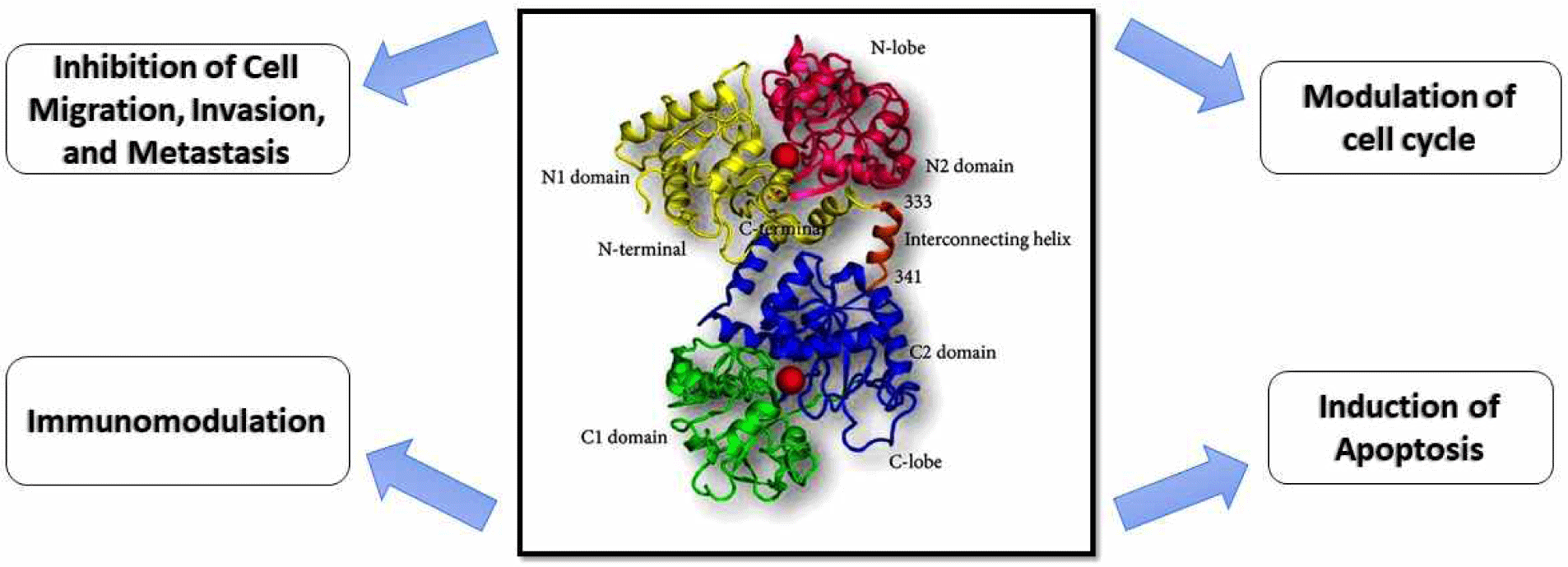
While the exact mechanism through which lactoferrin exerts its anticancer effects is still not fully clarified, its potential is linked to immunostimulant, intracellular and extracellular actions (Fig. 1). Lactoferrin’s extracellular effects are associated with the interaction with membranes and bind to membrane receptors [15], whereas its intracellular effects involve cell cycle arrest and apoptosis [8].
The induction of apoptosis includes the engagement of multiple extrinsic and intrinsic pathways. The extrinsic pathway is activated by ligands binding to death receptors, while the intrinsic pathway is initiated by oxidative stress, hypoxia, and DNA damage, which activates various pro-apoptotic proteins that influence cell fate [16,17]. Additionally, these regulators of apoptosis are affected by insulin signalling, JNK, and ERK signalling. In cancer, genetic changes prompt a rise in proliferation and invasive traits, disrupting the regulation of extrinsic and intrinsic pathways, and upsetting the balance between pro-apoptotic and anti-apoptotic factors, thus enabling the cells to escape apoptotic signals [14]. Resistance to programmed cell death is a key characteristic of cancer development. Biomolecule-derived chemotherapeutic agents may assist in restoring the apoptotic response. Lactoferrin has been shown to activate apoptotic signalling across diverse cancer types. In studies utilizing the stomach cancer cell line SGC-7901, bovine lactoferrin (bLf) effectively induced apoptosis by downregulating the AKT pathway [18]. Treatment with bLf resulted in the dephosphorylation of AKT at Ser473 and Thr308, thereby inhibiting downstream apoptotic regulators. Lactoferricin B, has also been identified to invoke ROS-dependent apoptosis in human leukaemia cell lines and other cancer models [19]. Another investigation showed that pro-apoptotic effects of bLf in animal models of colon cancer by enhancing the Fas signalling pathway [20].
Post-treatment with bLf, levels of Fas protein, caspase 3, and caspase 8 increased, confirmed by immunohistochemistry revealing apoptotic and Fas-positive cells in the colon. In a rat study, the same authors observed elevated levels of pro-apoptotic proteins Bax and Bid at the tumour site following bLf treatment [20]. Moreover, both isoforms of bLf, including apo and holo, were found to inhibit survival pathways frequently overexpressed in various cancers, binding to pro-apoptotic proteins and thereby obstructing apoptosis. Gibbson et al. [21] noted that isoforms of bLf prompted apoptosis in breast cancer cell lines, but normal breast cell lines showed no such effect.
Modulation of Cell Cycle
Various proteins, such as cyclin-dependent kinases are critical in regulating the cell cycle, with their inhibitors being essential for cell cycle progression. Proper regulation of cell cycle activity is vital, as any dysregulation may cause to the development of cancer. Lots of anticancer matters are designed to halt the cell cycle, thereby inducing cytotoxic effects in cancer cells. However, some chemotherapy drugs may lack specificity, impacting normal and cancer cells and resulting in side effects. In contrast, lactoferrin has emerged as a selective agent that targets cancerous tissues, inhibiting tumour cells and stimulation of normal cells growth. The mechanism by which both bovine and human lactoferrin supports normal cell growth involves the shortening of the cell cycle through the enhancement of mRNA expression of the proliferative cell nuclear antigen, thereby increasing cell populations in the G2 and S phases [22,23]. Additionally, bovine lactoferrin has been shown to stimulate cell growth via mitogen-activated protein ki nase (MAPK) and extracellular signal-regulated kinase (ERK) phosphorylation [24,25].
Regarding tumour cells, bovine and human lactoferrin have been proved to inhibit cell growth at various stages of the cell cycle. Researches demonstrated that bovine lactoferrin selectively impeded the growth of tumours in four breast cancer cell lines while not affecting normal cell lines. The study revealed that the mechanism behind cell cycle arrest involved the upregulation of phosphorylated AMPK and downregulation of mTOR which are critical for cell survival [24]. Another study indicated that bovine lactoferrin induced cell cycle arrest in oral carcinoma cells by increasing levels of the CDK inhibitor p21 and decreasing cyclin D1, which is integral for the progression through the G1 phase of cell growth [13].
Immunomodulation
The tumour microenvironment is largely infiltrated by inflammatory cells, particularly leukocytes such as macrophages, lymphocytes, dendritic cells and neutrophils. These cells release various inflammatory mediators, cytotoxic molecules, and soluble factors that influence cancer progression [26]. The interaction between cancer and immune responses often determines the fate of tumors. Numerous studies have highlighted the carcinogenic potential of leukocytes through the secretion of IL-1β and IL-6, which are elevated in different cancer models [27]. However, specific leukocyte subtypes, including cytotoxic T cells and natural killer cells with anti-tumor properties [27]. Consequently, the immune response can have dual roles in cancer, engaging in both pro-cancerous and anti-cancerous activities, depending on the equilibrium between innate and adaptive immunity. This interaction in immunomodulation plays a main role in cancer biology, suggesting that molecules enhancing cytotoxic immune components are promising candidates as adjuvants to chemotherapy. Lactoferrin has been shown to boost certain aspects of adaptive immunity while also exhibiting anti-inflammatory properties [28]. Both bovine and human lactoferrin have been reported to penetrate the host cell nucleus and interact with DNA, thereby affecting gene expression, managing inflammation, and regulating tumor growth. In studies conducted on mice, the oral administration of bovine lactoferrin demonstrated anti-tumor effects by elevating mucosal IL-18 mRNA levels in the small intestine [29]. Furthermore, it has been documented that bovine lactoferrin can inhibit tumor growth in IFN-γ knockout mice by activating the IFN-α/IL-7 pathway [29]. In another research effort, bovine lactoferrin was reported to hinder the growth of tumors in human lung cancer cells and mouse models by modulating growth factor related to vascular endothelial levels. The results revealed that bovine lactoferrin reduced VEGF levels in cancer cells and lowered the expression of pro-inflammatory cytokines [30]. In addition to its direct influence on immune function, bovine lactoferrin has been found to regulate reactive oxygen species levels by binding to free iron—an important contributor to ROS production [31]. Moreover, bovine lactoferrin has shown protective effects against iron-related disorders that can cause to cancer by modulating immune responses and decreasing levels of pro-inflammatory cytokines like tumour necrosis factor and interleukins [31].
Lactoferrin as a Carrier for Drug Delivery in Cancer
In cancer treatment, various compounds are being investigated for their ability to eliminate cancer cells but saving normal tissues. Although many of these substances show promising anticancer properties, they frequently lack the desired specificity, leading to cytotoxic effects on healthy cells and contributing to multi-drug resistance in cancer therapies [32]. The non-targeted delivery of chemotherapy agents is critical in the resistance development, making precise targeting of cancer cells essential in cancer therapy. A range of agents, such as antibodies, organic compounds, nanoparticles, and lactoferrin, has been evaluated for their ability to selectively target cancer cells [33]. Notably, lactoferrin has demonstrated the capability to form conjugates with nanoparticles that carry anticancer drugs, aiding in targeted delivery.
Doxorubicin (Dox), a commonly used chemotherapy drug, has been studied in conjunction with bovine lactoferrin to enhance absorption in prostate cancer cell lines and mouse models. The results showed that the conjugation of Dox with bovine lactoferrin significantly increased Dox-induced cytotoxicity and helped retain the drug within the cancer cells [34]. Moreover, the bovine lactoferrin-Dox complex effectively overcame multi-drug resistance in resistant cancer cells. In an in vivo cancer model, the oral administration of this complex led to improved survival rates and significant tumour reduction in mice. Importantly, the complex exhibited lower toxicity compared to standalone Doxorubicin treatment, as it reduced the overall toxicity associated with Doxorubicin while increasing serum amount of TNF-alpha, CCL4, and IFN-gamma [34].
Glioblastoma, a highly aggressive type of melanoma affecting the central nervous system, frequently remains untreated due to the challenges faced by chemotherapeutic agents in reaching glioma cells. Many agents cannot penetrate the blood-brain barrier, and those that can may not accurately target the intended site, resulting in significant complications. Lactoferrin, known for its ability to cross the blood-brain barrier, has a favourable safety profile. Lactoferrin-derived nanoparticles loaded with diverse anticancer agents are safe and enhance the penetration of the blood-brain barrier, ensuring effective delivery of chemotherapy to glioma cells [35].
Liposomal formulations incorporating lactoferrin have been studied for their potential as anticancer agents in drug delivery applications. Specifically, polyethylene glycol (PEG)-modified liposomes containing bovine lactoferrin have been employed to deliver Doxorubicin in various cell lines and animal models of liver cancer [12]. The Dox-loaded bLF-PEG-Liposomes demonstrated increased cellular uptake of Dox and significantly inhibited tumour cell growth in mouse liver cancer xenografts. This complex was also administered intravenously in breast cancer models, where it exhibited enhanced accumulation at the tumour site, leading to improved tumour suppression compared to PEG-liposomes containing only Dox [24]. These findings highlight the role of bovine lactoferrin in effective drug delivery and tumor suppression. Similarly, micellar formulations featuring bLF have been used for the targeted delivery of rapamycin, inducing selective death of cells in breast cancer [36]. The biocompatibility and serum stability of bLF-micelles enhance rapamycin’s targeting and cytotoxicity toward tumor cells [36]. This evidence positions bovine lactoferrin as a promising candidate for the specific delivery of chemotherapeutic agents, potentially addressing the challenges of multi-drug resistance in cancer treatment.
Prostate Cancer
Prostate cancer ranks as a leading malignancy and is the second most prevalent cancer in men. Frequently diagnosed at advanced stages due to the absence of early symptoms. There is an increasing interest in non-toxic and natural treatments for prostate cancer. The effects of bovine lactoferrin on PC-3 cells exhibit high metastatic potential in prostate cancer [37]. These cells display V-ATPase, which facilitates cancer invasion, metastasis, and an acidic tumour microenvironment.
Zadvornyi et al. [38] explored the impact of exogenous lactoferrin on the invasiveness of DU145 and LNCaP prostate cancer cells. Human prostate cancer cells were cultured with lactoferrin in a dose-dependent manner, after which their invasiveness was evaluated. Lactoferrin led to 40% reduction in invasiveness for DU145 cells and 30% reduction for LNCaP cells. Lactoferrin also enhanced the expression of tumour-suppressive microRNAs (miR-200b and miR-133a) In DU145 cells, indicating its potential to alter the phenotypic characteristics of prostate cancer cells and influence critical cellular processes associated with growth [38]. In a separate investigation, PC-3 metastatic cancer cells were treated with bovine lactoferrin (bLF), which led to apoptosis, cell proliferation, intracellular pH, and extracellular acidification. The findings revealed that bLF inhibited proliferation of cells and induced cell death in PC-3 cells while also decreasing the rates of intracellular acidification. Control BJ-5ta cells exposed to the same treatment showed no sensitivity to bovine lactoferrin, underscoring its specificity towards cancer cells. The authors proposed that the observed inhibition in cancer cells may be attributed to the suppression of V-ATPase, a factor associated with cancer metastasis. These results indicate that milk lactoferrin could be an effective agent in managing prostate cancer and inhibiting its spread [38].
Breast Cancer
The anticancer effects of lactoferrin derived from goat colostrum were assessed across several cancer cell lines in the human [39]. Caprine lactoferrin was isolated using ultrafiltration and chromatographic techniques. After purification, it was tested against five cancer cell lines, including those from breast, colon, stomach, lung, and cervical cancers. The results demonstrated that caprine lactoferrin significantly inhibited the proliferation of cancer cells in a dose-dependent manner, resulting in only 20%–30% cell viability compared to normal cells. Particularly, ZR‐75‐1 breast cancer cells showed the most significant growth reduction, with an IC50 value of 27.5 μg/mL, indicating that milk lactoferrin is a powerful against breast cancer. Duarte et al. [5] examined the bovine milk lactoferrin on cell lines of human breast cancer. These cells treated with 0.125 to 125 μM lactoferrin. The results showed that bovine lactoferrin (bLF) decreased the viability of T47D and HS578T cells by 54% and 47%, respectively. Moreover, the proliferation rates for T47D and HS578T cells were reduced by 63.9% and 40.3%, respectively. A decrease in cancer cell migration was observed only in T47D cells, while apoptosis increased in both cell lines. In another investigation, Gibbons et al. [40] evaluated the cytotoxicity and invasive characteristics of both iron-saturated and iron-free forms of bovine lactoferrin on cell lines of breast cancer; MCF-7 and MDA-MB-231 in the human. The findings indicated that the iron-free form exhibited superior cytotoxicity towards MCF-7 and MDA-MB-231 cells in a dose-dependent manner, with no cytotoxic effects noted in normal breast cells. Both forms of bLF significantly impacted the invasion capabilities of cancer cells. Critically, the survivin protein, commonly elevated in breast cancer cases, was inhibited by both forms of bLF. Additionally, bovine milk lactoferrin was found to modulate several apoptotic molecules, such as p53 and Bcl-2 proteins [40].
Colorectal Cancer
Bovine lactoferrin and lactoferricin B (LFcinB), have shown promising anticancer effects on colorectal cancer cells, believed to act through the regulation of various signalling pathways. Studies involving HT-29 colorectal cancer cells indicated that these agents triggered apoptosis in HT-29 cells, while sparing normal intestinal cells [41]. A cell viability assay revealed a reduction in the saving of cancer cells of colorectal following treatment. Also, bLF and LFcinB were shown to influence p53 and angiopoietin signalling pathways, with protein expression analyses indicating increased levels of p53, p21, and caspase 8 in HT-29 cells after treatment [41]. Colorectal polyps, which are abnormal growths in the colon lining, can progress to colon cancer if left untreated. A randomized controlled trial assessed the impact of orally administered bovine lactoferrin on the growth of colorectal polyps [42].
The effectiveness of bovine lactoferrin and lactoferricin was also evaluated on the human colorectal cancer cell line CaCo-2 [43]. Treatments with bLF and lactoferricin resulted in decrease cell proliferation. Cell cycle analysis demonstrated that bLF extended the S phase and lowered cyclin E1 levels, thereby reducing cell proliferation. Furthermore, the therapeutic effects of camel milk lactoferrin on HCT-116 colorectal cancer cells, significantly inhibited cancer cell proliferation and lessened DNA damage [43].
In a study with 104 participants, those who received 3 g of bLF daily for 12 months saw significant reductions in colorectal polyp growth compared to a placebo group, which received 1.5 g for the same duration. This indicates that oral administration of 3 g bLF could significantly aid in the prevention of colorectal cancer by effectively reducing polyp growth. While polypectomy can remove existing polyps, it does not eliminate the risk of developing new ones. Thus, this study suggests that oral bovine lactoferrin could serve as a supplementary treatment in managing colorectal polyps and preventing colorectal cancer [43].
Lung Cancer
Bovine lactoferrin, a milk-derived component, has recently emerged as a promising chemo preventive agent for lung carcinoma progression. Bovine lactoferrin (bLF) was assessed for its efficacy [30]. The human lung cancer cell line A549, known for its high expression of growth factor of vascular endothelial; a key marker for lung cancer—was used for in vitro evaluations. For the in vivo study, a transgenic mouse expressing the human VEGF-A165 gene, which is prone to developing pulmonary tumours, was utilized. Both experimental setups showed that bLF effectively prohibited the proliferation of lung cancer cells in a dose-dependent way [30]. Another study explored the impact of bovine lactoferrin on oesophageal and lung carcinogenesis in rat models [44]. This research indicated a tendency toward reduced papilloma formation in the oesophagus and a substantial decrease in the size of larger papillomas among rats administered bLF. Furthermore, the incidence of tumour (carcinomas and adenomas) in the lungs was significantly lower in the treated animals [44].
Interestingly, bLF treatment also led to a reduction in the expression of pro-inflammatory and anti-inflammatory cytokines, such as TNF-alpha and interleukins (IL-4, IL-6, IL-10). This decline in cytokine expression may have played a role in mitigating inflammation, thereby limiting lung cancer cell proliferation. Following bLF treatment, the expression of vascular endothelial growth factor was downregulated as well. Additionally, in the in vivo model with elevated human VEGF-A165 expression, tumour growth was suppressed, and VEGF-A165 expression in the mice decreased. Thus, it can be concluded that bovine lactoferrin holds significant promise as a therapeutic agent against lung carcinoma by inhibiting inflammation and angiogenesis [44].
Bladder Cancer
Research demonstrated that a 2% concentration of bovine lactoferrin reduced tumour multiplicity and was linked to decreased cell proliferation in N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN)-induced bladder carcinogenesis in rats. This effect may be attributed to its direct action in the urinary tract. The chemo preventive potential of bovine lactoferrin, abundant in colostrum, was studied in a rat bladder medium-term bioassay [45].
As a natural defence protein abundant in various bodily secretions, bovine lactoferrin possesses properties that inhibit carcinogenesis and tumour growth. Kanwar et al. evaluated iron-saturated bovine lactoferrin’s ability to enhance chemotherapy in lymphoma treatment [46]. C57BL/6 mice were fed diets supplemented with either iron-saturated or naturally occurring bovine lactoferrin with lower iron levels, followed by subcutaneous inoculation with tumour cells and subsequent chemotherapy. The results showed effective eradication of large EL-4 lymphomas (0.6 cm) in mice treated with iron-saturated bovine lactoferrin in the six weeks before chemotherapy, whereas it did not have the same impact in those receiving iron-free bovine lactoferrin. Notably, the study emphasized that the efficacy of bovine lactoferrin in enhancing chemotherapy responses is contingent upon its iron-binding capacity.
Lactoferrin and Oral Squamous Cancer
Chea et al. [13] examined the anticancer effects of bovine lactoferrin on oral squamous cancer cells. Their study involved four cell lines (HSC2, HSC3, HSC4, and normal RT7 cells) treated with bLF at concentrations of 1, 10, and 100 μg/mL. All concentrations of bLF noticeably prevented induced apoptosis and cell proliferation. Flow cytometry and western blot analyses revealed that the molecular mechanisms of bLF are significant in managing oral squamous cancer. The study also demonstrated that bLF activates p53, which is associated with apoptosis induction and cell cycle arrest in the G1/S phase in oral squamous cancer cells. Furthermore, western blot analysis indicated that bLF downregulated Akt phosphorylation and the cytokine signalling suppressor, suggesting that bLF can modulate various signalling pathways, including JAK/STAT and mTOR signalling. These findings imply that bovine lactoferrin may be beneficial in managing oral squamous cancer cells.
Lactoferrin and Leukaemia
Lactoferricin B, a peptide fragment obtained from bovine lactoferrin, is created through the hydrolysis of bovine lactoferrin by acid pepsin. In vitro treatment with lactoferricin B quickly induced apoptosis in several human leukaemia and carcinoma cell lines. The findings indicated that lactoferricin B inhibits cancer cells by activating the mitochondrial pathway of apoptosis, primarily through the generation of reactive oxygen species. Upon treatment with lactoferricin B, Jurkat T leukaemia cells produced reactive oxygen species, which led to a caspase-2-mediated loss of mitochondrial transmembrane potential, ultimately activating caspase-9 and caspase-3. Furthermore, Jurkat T leukaemia cells that overexpressed Bcl-2 demonstrated reduced sensitivity to the apoptosis induced by lactoferricin B, as evidenced by mitochondrial swelling and the release of cytochrome c to the cytosol [8].
Anti-Inflammatory Effects
Significant research over the past decade has concentrated on the anti-inflammatory efficacy of lactoferrin which showed by inhibiting the release of cytokines that facilitate the recruitment and activation of immune cells at inflammation sites. Studies have shown that bovine lactoferrin affects cytokine in splenocytes of obstructive jaundiced rats [47]. Additionally, lactoferrin has been found to increase the inflammatory cytokines IL-4 and IL-10 in colitis rat [48]. Research on camel lactoferrin suggested its role in alleviating rheumatoid arthritis by inhibiting of signalling pathway of the mitogen-activated protein kinase in arthritic rats [49], resulting in decrease of TNF-α and IL-10, and protein expression of NF-κBp65, COX-2, and iNOS. Moreover, the interaction of lactoferrin with lipopolysaccharide (LPS) has been linked to its ability to downregulate pro-inflammatory cytokine expression through its lactoferrin C (Lfc) domain [50]. Notably, lactoferrin can neutralize the effects of LPS [51] by competing with LPS-binding protein to LPS, thereby preventing the transfer of endotoxin to mCD14 on macrophage surfaces [50]. Additionally, lactoferrin has been shown to reduce hydrogen peroxide generation resulting from the interaction of LPS with the L-selectin on neutrophils [52]. Beyond its binding with CD14 and LPS, lactoferrin exhibits other mechanisms that inhibit pro-inflammatory mediators. The internalization of lactoferrin by monocytic cells has been associated with a decrease in production of TNF-α-stimulated IL-6 [51], from the inhibition of NF-kB binding to the TNF-α promoter [53]. Furthermore, the anti-inflammatory effects of lactoferrin on B cells are attributed to interaction with CpG-containing oligonucleotides [54]. In rat models of arthritis induced by adjuvants, bovine lactoferrin was reported to suppress TNF-α while enhancing IL-10 production [55]. Recombinant forms of lactoferrin derived from human and bovine milk have also been characterized as inhibiting LPS-mediated preterm delivery in murine models by blocking IL-6 expression [56].
Pro-Inflammatory Efficacy of Lactoferrin
Na et al. [57] declared that under certain conditions, the lactoferrin and LPS complex can stimulate inflammation-related species in the macrophages. However, Sorimachi et al. [58] reported numerous studies indicate that lactoferrin can activate macrophages, resulting in the release of TNF-α, IL-8 and nitric oxide. Treatment with the LPS and lactoferrin has been shown to induce tolerance to LPS in cells [57]. Additionally, lactoferrin may restore humoral defence mechanisms and enhance production of IL-6 in cyclophosphamide-immunocompromised mice through peritoneal and alveolar cells [59,60]. While TLR-4 is essential for optimal lactoferrin-induced CD40 expression, it does not play a role in the IL-6 production from murine peritoneal cells elicited by bovine lactoferrin. Furthermore, another study highlighted that TLR-4 is critical for initiating an anti-viral condition in host cells against vesicular stomatitis virus, but it is not required for TNF-α production triggered by lactoferrin [61]. In induced immune compromise murine by cyclophosphamide, lactoferrin was found to enhance T cell-derived immune responses by replenishing the T cells, leading to an increase in CD3+ T cells and CD4+ T cells [59]. Recent evidence suggests that oral intake of lactoferrin in murine models infected with type 1 herpes simplex virus can amplify the cytokine response and mitigate body weight loss [62]. Pepsin hydrolysates from bovine lactoferrin have also been shown to promote IL-18 production in epithelial cells of murine small intestine, consequently fostering the expression of various immune-related genes, such as IFN-g and other pro-inflammatory mediators [63]. Hence, bovine lactoferrin may also inhibit metastasis and carcinogenesis. It has been demonstrated to obstruct angiogenesis by elevating serum IL-18 levels and impairing endothelial function [64]. Continuous injections of bovine lactoferrin can create a Th1-cytokine-dominant environment in the peripheral blood of chronic hepatitis C cases, potentially assisting in the clearance of chronic HCV in conjunction with IFN treatment [65]. In lactoferrin-treated murine models, a marked increase in TNF-α and IFN-g was seen in comparison with untreated groups upon stimulation with Candida albicans killed by heat from cervical lymph node cells [66].
Lactoferrin and Adaptive Immune Responses
Lactoferrin plays a vital role in enhancing immune responsiveness, particularly concerning its effects on B cells. Research indicates that lactoferrin can facilitate the maturation of immature B cells in mice, as evidenced by increased production of complement 3 receptors and enhanced acquisition of IgD on B cell surfaces. When cultured with lactoferrin, immature B cells demonstrated a heightened ability to stimulate T-cell proliferation in response to specific antigens, indirectly indicating improved B-cell antigen presentation [67]. T cell activation occurs through antigens presented by B cells, which is crucial for the release of cytokines necessary for isotype switching. Oral administration of lactoferrin has been shown to enhance the release of both IgG and IgA from murine Peyer’s patches [68], with notable increases in antibody titers observed in both intestinal secretions and serum [69].
Receptor sites specific to lactoferrin have been identified on all T cell subsets, including δγ T-cells [70]. Studies on human and bovine cases revealed lactoferrin receptor sites present on the T-cell line Jurkat [71], with lactoferrin interacting via receptor-facilitated endocytosis. The impact of lactoferrin on T lymphocytes can be different, often related to the state of differentiation, maturation, and activation of the T cells. Human milk-derived lactoferrin stimulates the maturation of murine CD4−CD8−T cells while promoting CD4 expression [72] through the activation of intracellular MAP kinase pathways via Erk2 in conjunction with p56lck [73]. Human lactoferrin enhances the expression of the T-cell receptor (TCR) complex component known as the human T-cell ζ-chain [74]. Lactoferrin exhibits a dual effect on T cell responses; it can decrease overall cytokine production when added to mitogen-activated T cells [47]. Both human and bovine milk-derived lactoferrin have also been linked to reduced IL-2 and IFN-γ production in cultured murine splenocytes [75]. Recombinant lactoferrin has been demonstrated to decrease T cell proliferation, IL-5 production, and CCR4 chemokine expression in a T cell activation model triggered by nickel [76]. Lactoferrin enhanced delayed hypersensitivity reactions mediated by T cell, as indicated by swelling in the footpad following the introduction of antigenic proteins such as sheep red blood cells, BCG, and ovalbumin [77,78]. The administration of lactoferrin suggests a mechanism that promotes adaptive cell reconstitution through proliferative pathways, emphasizing its role in restoring humoral defense mechanisms in immune-compromised individuals [59,60]. The effects of lactoferrin on T cells can be more specifically described in relation to targeted cellular subsets. It can influence the balance between TH1 and TH2 immune responses by modulating T-cell-secreted cytokines, including IL-4, IFN-γ, and IL-5. Research on camel milk lactoferrin indicates that it can enhance the balance of Th1/Th2-mediated cytokine production during hepatocyte damage [79]. Notably, oral administration of lactoferrin increases TH2-mediated T-cell cytokines in mice, while mitogen-stimulated splenocytes and intramuscular lactoferrin administration boost TH1-related T-cell cytokines [69]. In summary, lactoferrin interacts with B cells, key antigen presenters, facilitating subsequent T-cell interactions that strengthen the antibody response.
Conclusion
Natural biomolecules have gained significant interest within the scientific community for their anticancer properties attributed to their high stability, biocompatibility, specificity, and minimal side effects. Identifying compounds with cancer-preventing attributes and treatment-enhancing capabilities is essential in this context. Bovine lactoferrin has been recognized for its anticancer potential across various cancer types, functioning through both extracellular and intracellular mechanisms. Moreover, it has emerged as a promising carrier for the targeted delivery of chemotherapeutic agents aimed at eliminating cancer cells. Lactoferrin has also been associated with the modulation of cellular death and inflammation-related damage. The lactoferrin receptors on diverse immune cells highlights the vital role of lactoferrin in modulating immune responses. Understanding the interactions of lactoferrin with its receptors is crucial for the development of future strategies to tackle inflammation-related disorders.