Introduction
Whey protein in milk has a variety of functional properties and has been recognized for its biological value. These functional properties are of particular interest in the food and pharmaceutical industries [1]. It has been reported that the major protein constituting whey protein plays an important role as a precursor of physiologically active peptides showing various effects such as α-lactalbumin and β-lactoglobulin cholesterol- lowering action. Contains immunoglobulins and serum albumin [2].
In particular, studies on the antioxidant activity and functionality of milk protein and whey-derived peptides hydrolyzed during the fermentation of lactic acid bacteria (LAB) are being conducted [3]. It has been shown that antioxidant activity varies depending on the amino acid sequence derived from the specificity of the whey-derived protein hydrolysate. According to the functional specificity of whey-derived protein hydrolyzate, it can be used as a natural antioxidant, and there is a study that it can inhibit the oxidation reaction in the food processing industry by using the property [4].
Hydrolysis of whey protein is one of the most useful methods for obtaining peptides. But whey-derived protein shows high resistance to hydrolysis and has hydrophobicity, so it is difficult to induce hydrolysis despite the high utility value of the precursor. As a solution to this problem, the production of peptides through the fermentation of whey-derived proteins using LAB is attracting attention [5]. Thereafter, strains are isolated and identified, and the utilization value is evaluated by confirming the hydrolysis utilization of whey-derived proteins and the substrate properties and functionality of the resulting peptides [6].
Hydrolysis of whey-derived proteins with lactobacilli produces some proteases that reduce the potential for allergic reactions seen in milk proteins [7-9]. As a result, LAB can produce a wide variety of peptides, and the method of standardizing this production process can be very difficult. This point is a factor to be overcome, but at the same time, it also shows the potential of hydrolysis of whey-derived protein using LAB. This approach can be considered for use in developing peptides that display novel traits and can explore the potential for industry-wide applications [9].
The objectives of this research were to hydrolyze whey-derived proteins using LAB in order to identify peptides with exceptional functionality and immunomodulatory effects and to investigate the potential application of these whey-derived proteolytic peptides in the food industry.
Materials and Methods
LAB were isolated from homemade kimchi which was collected from different regions in Korea. Screening of selected strains was performed by colony shape on the plates, gram staining, and observation of strain morphology using a microscope. Selected strains were confirmed by 16S-rRNA gene sequencing. The 16S-rRNA gene sequences were analyzed by the Gene Bank database (Macrogen, Korea), and identification was performed based on the 16S-rRNA gene sequence homology.
Whey protein concentrate (WPC, 80% protein) was obtained from Hilmar Ingredients (USA). 7%(w/v) whey protein and 3%(w/v) glucose (Sigma-Aldrich, USA) were added at a concentration for the growth of LAB. Adjusted to the optimum active pH of the LAB with 1 N NaOH (Sigma-Aldrich). The whey protein solution was heat treated at 65°C for 30 min [10].
In order to obtain peptide fractions from whey protein hydrolysates, gel filtration using a preparative chromatography system was performed (Waters, USA). The whey protein hydrolysates dissolved in 5 mM sodium phosphate buffer with 0.15 M NaCl (Sigma-Aldrich), pH 7.0. It was then filtered through a PVDF 0.45 μm sterile syringe membrane filter (Futecs, Korea). Next, the whey protein hydrolysates loaded 3.0 mL on the Hiprep 16/60 Sephacryl S-100 HR column (GE Healthcare Life Sciences, USA) and then eluted at a flow rate of 1.0 mL/min. A detector was set at 280 nm (Table 1).
The ABTS radical cation scavenging activity of the ethanol extract was measured according to the method [11]. ABTS was dissolved in ethanol to make a 7 mM ABTS stock solution. This radical cation was produced by reacting the ABTS stock solution with 2.45 mM potassium persulfate and by leaving the mixture for 16 hr until the reaction was completed and the absorbance was stabilizing. The ABTS stock solution was diluted in ethanol to an absorbance of 0.7±0.02 at 734 nm. After adding 0.9 mL of the diluted ABTS solution to 0.1 mL of each fraction and mixing them, the absorbance was taken 3 min later [12]. Results were expressed in units of Trolox equivalent capacity in units of mM.
The DPPH free radical scavenging activities were measured by using the method reported by [13] with slight modification. First, 0.2 mM DPPH solution was prepared for use in the experiment by mixing DPPH (Sigma-Aldrich) and ethanol (Samchun, Korea).
Thereafter, DPPH solution and WPCH samples were mixed in a 1:1 ratio (v/v) and reacted in a dark room at room temperature for 30 minutes. The absorbance was measured at 570 nm, results were expressed in units of Ascorbic acid (Sigma-Aldrich) equivalent capacity in units of mM.units of Ascorbic acid (Sigma-Aldrich) equivalent capacity in units of mM.
The Hydroxyl radical scavenging activity of each fraction was measured based on the report of [14] with some modifications. The 0.75 mM 1,10-phenanthroline (Sigma- Aldrich), Phosphate buffer (pH 7.0), 2.5 mM FeSO4 (Sigma-Aldrich), and whey protein hydrolysate fraction samples were mixed in a ratio of 1:1:1:1 (v/v). The 20 mM Hydrogen peroxide (Junsei Chemical, Japan), the reaction reagent was finally mixed in the same ratio (v/v). The reaction was carried out in a water bath at 37°C for 90 minutes. The absorbance was measured at 593 nm. The results were expressed in units of Ascorbic acid (Sigma-Aldrich) equivalent capacity in units of mM.
Cell viability was determined by MTT assay as previously reported by [15,16]. RAW 264.7 cells were seeded in 96-well cell culture plates for 24 hr. After removing the medium, RAW 264.7 cells were treated with various concentrations of fraction samples in a serum-free medium for 24 hr. After that, RAW264.7 cells were treated with MTT (5 mg/mL) for 2–3 hr and re-incubated in an incubator (37°C, 5% CO2). After culture, supernatants were removed, The dark blue formazan crystals formed in intact cells were extracted with DMSO and the absorbance at 540 nm was measured with a microplate reader (BioTek, USA). Cell viability (%) was expressed in the treatment to control. The viability of cells was calculated considering controls as 100% viable.
RAW 264.7 cells were seeded in 96-well cell culture plates at a density of 5×104 cells/well and incubated for 16–18 hr. The amount of NO was calculated by measuring the amount of nitrite, an oxidized product, in the cell culture supernatants as previously explained. After removing the medium, RAW 264.7 cells were treated with various concentrations of fraction samples in the medium for 2–3 hr. Thereafter, lipopolysaccharide (LPS) at a final concentration of 100 ng/mL was treated and stimulated in the same volume in the medium for 20–22 hr. Griess reagent was added to the supernatant in a ratio of 1:1 (v/v). The absorbance at 540 nm was determined in a Microplate reader after 15 min at room temperature [17].
RAW 264.7 cells were seeded in 96-well plates at a concentration of 5×104 cells/mL for 24 hr. After removing the medium, RAW 264.7 cells were treated with various concentrations of fraction samples in a serum-free medium for 2–3 hr. After that, LPS at a final concentration of 100 ng/mL was treated and stimulated in the same volume in a serum-free medium for 20 hr. Cell-free supernatants were collected and the cytokine content was measured by a Mouse IL (interleukin)-1, IL-6, and Mouse tumor necrosis factor (TNF)-α ELISA kit (Komabiotech, Korea) using an ELISA. The optical density of the microplate was read at 450 nm.
Amino acid profiling proceeded according to Waters amino acid analysis AccQ-Tag manual. Waters AccQ-Tag·Fluor reagent kit was used for the derivatization of the sample and standard. Amino acid standard (Sigma-Aldrich) was used as a standard. The range of sample amount was 0.02–0.08 μg (20–1,000 pmol). The other system conditions are shown in Table 2 below.
Results
After the isolation of LAB from kimchi, strain identification was performed using 16s rRNA sequencing. DK209 was identified as Lactobacillus paracasei (99% similarity to GeneBank sequence).
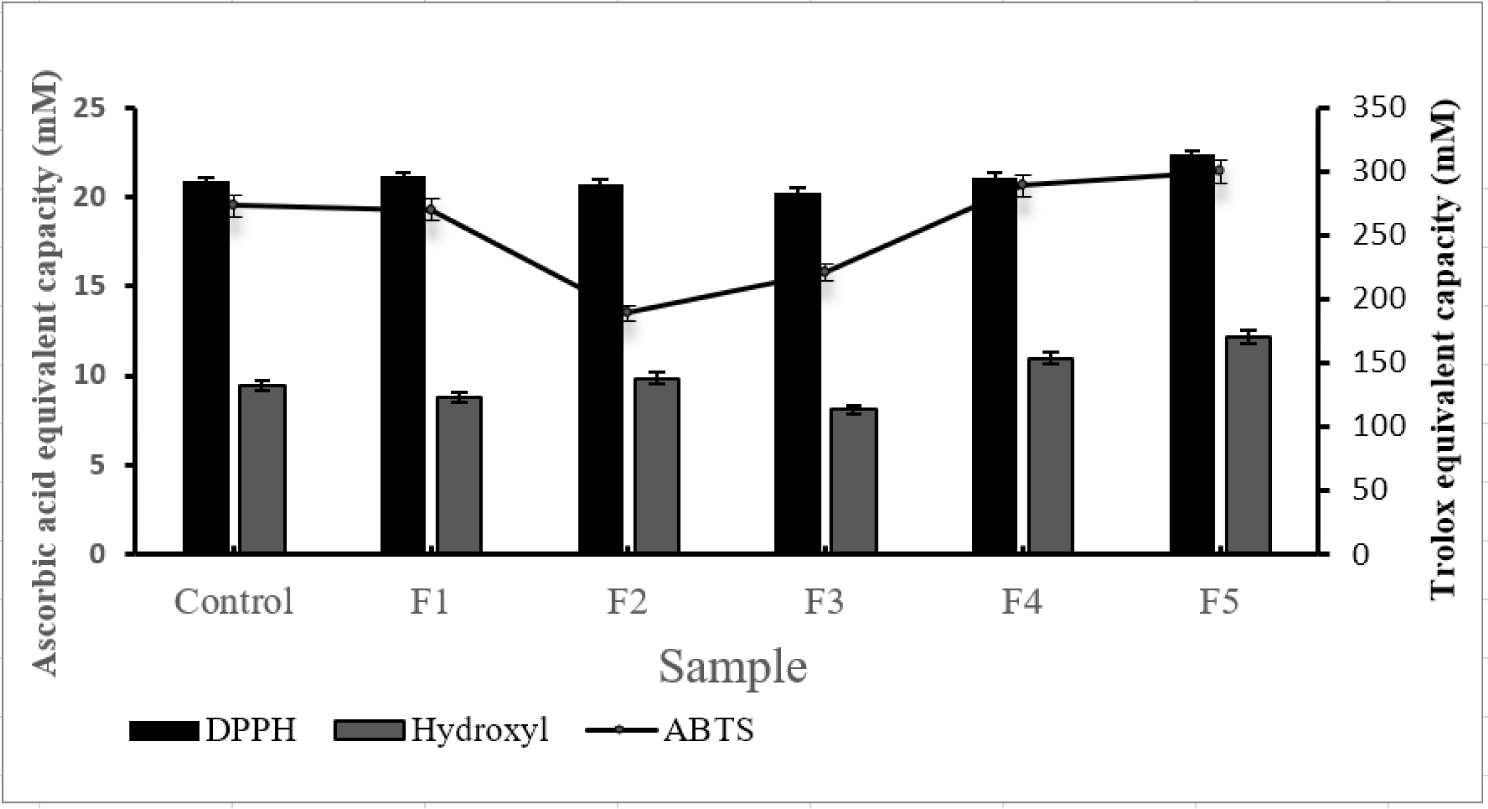
To verify antioxidant activity, three antioxidant assays (ABTS, DPPH, and Hydroxyl radical scavenging) were conducted. The F5 sample showed superior antioxidant activity compared to the control, which was confirmed through cross-validation (Fig. 1).
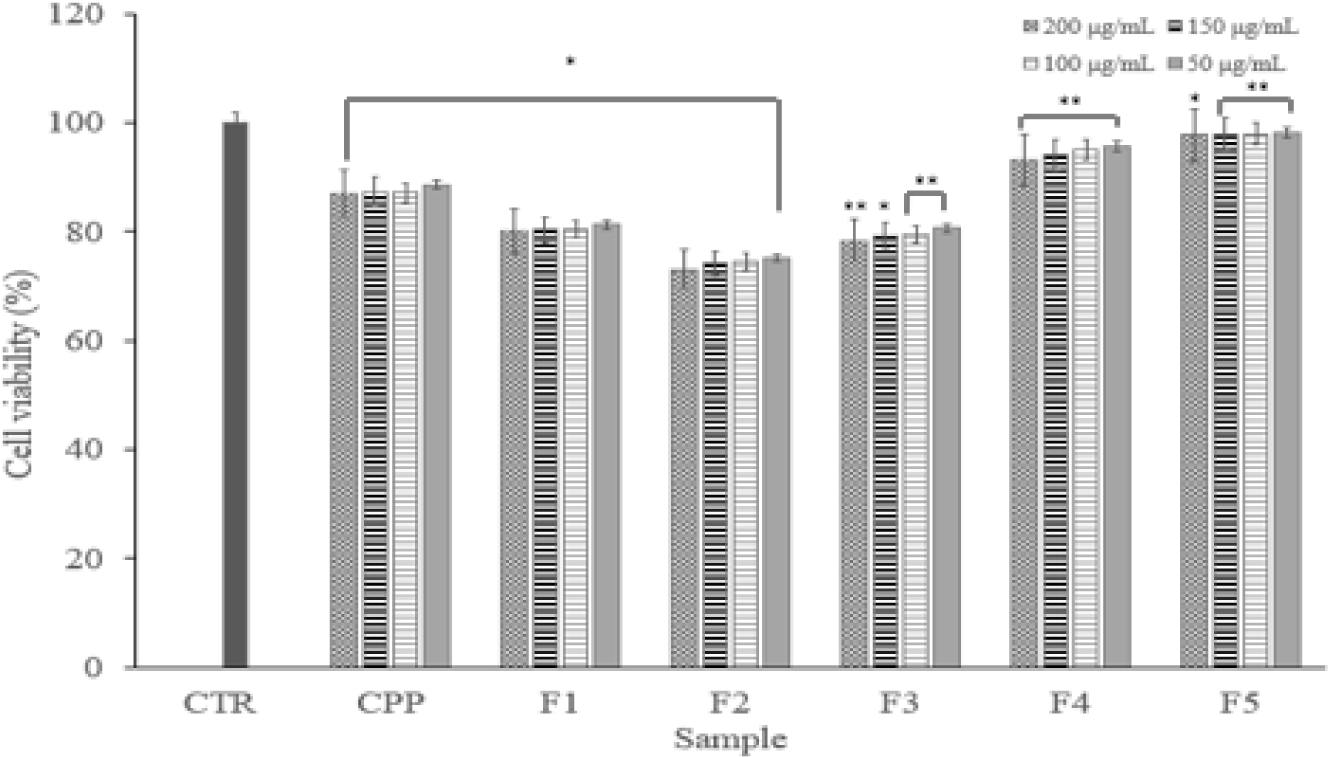
Cytotoxicity test via the MTT assay is widely used in vitro toxicology experiments. According to the experimental results, despite the increase in concentration, there was no significant difference by concentration. There was no significance on concentration differences and cell death. In addition, it showed cell viability of 80% or more. Therefore, it was confirmed that there is no cytotoxicity of fraction samples on macrophage (Fig. 2).
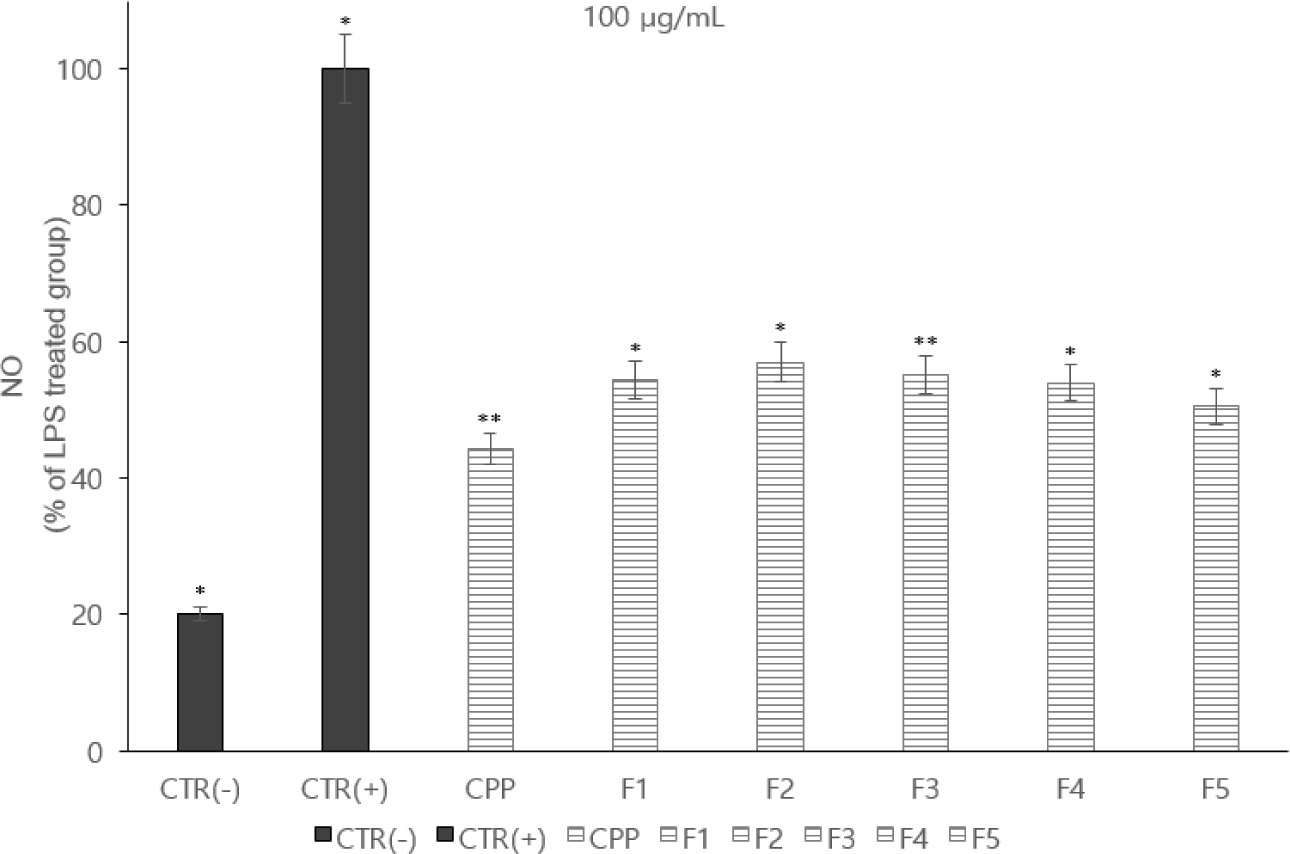
NO plays an important role in fluid coagulation and immune function in the body. However, like the mechanism of reactive oxygen species, it is oxidized and converted into active NO. This creates an oxidizing agent that is directly toxic to cells. NO, produced by cells that are inflamed, is increased in tissue damage or in inflammatory diseases [18]. As a result of the study, NO production was lower in F5 than in other fractions. These points can have a functionally positive effect on inflammation or cellular immunity (Fig. 3).
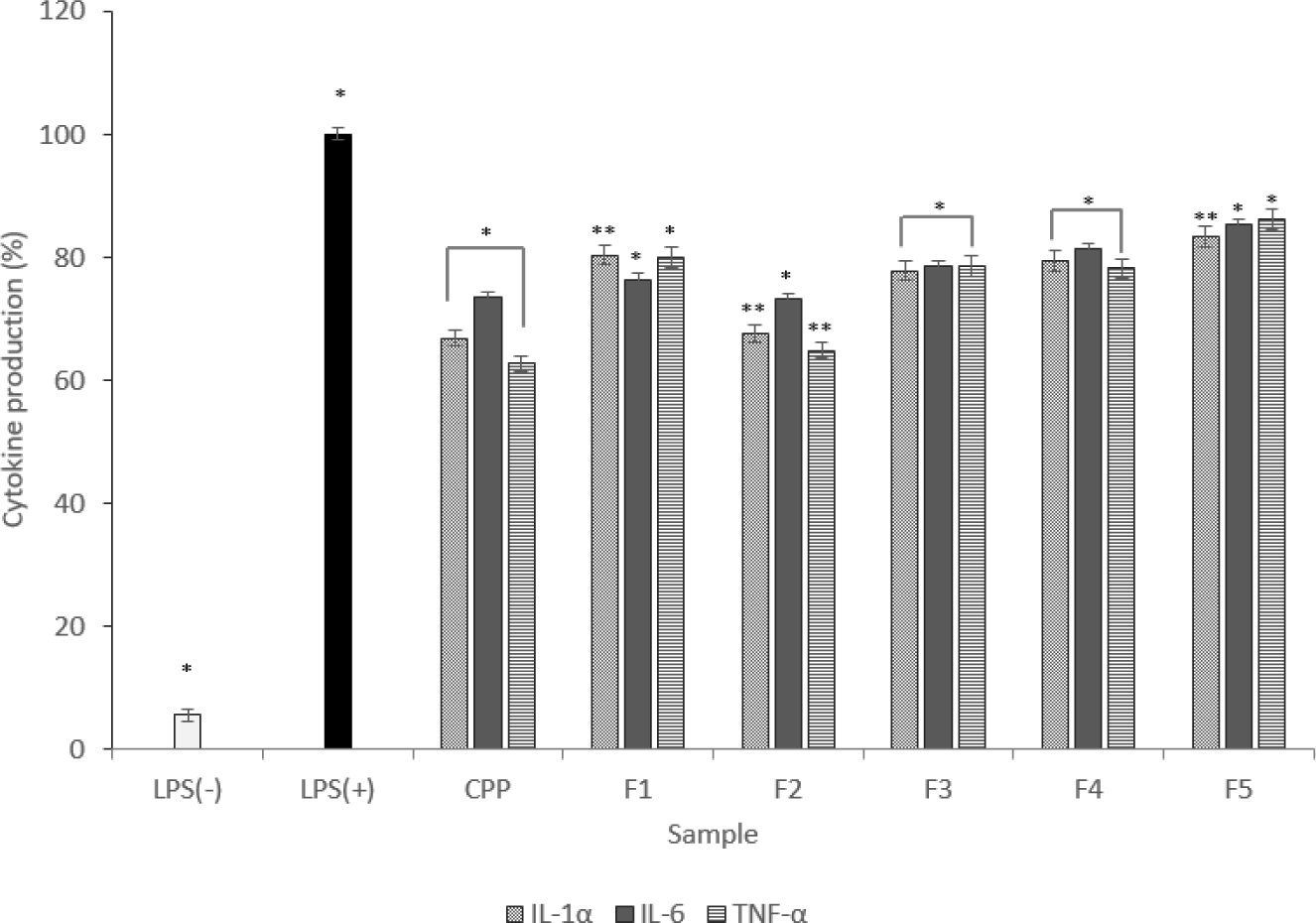
Immune diseases are in many parts associated with cellular inflammation [18]. In this experiment, IL-1α, IL-6, and TNF-α were measured, and the expression levels were shown in a graph. For cytokine measurement, cytokine measurement results using ELISA (IL-1α, IL-6, TNF-α) The production was significantly lower than that of the LPS(+) group.
Therefore, the anti-inflammatory of the hydrolyzed fraction can be inferred. And due to the function of macrophages for each fraction, the result of low expression of TNF-α indicates that the activation of pro-inflammatory cytokines is inhibited (Fig. 4).
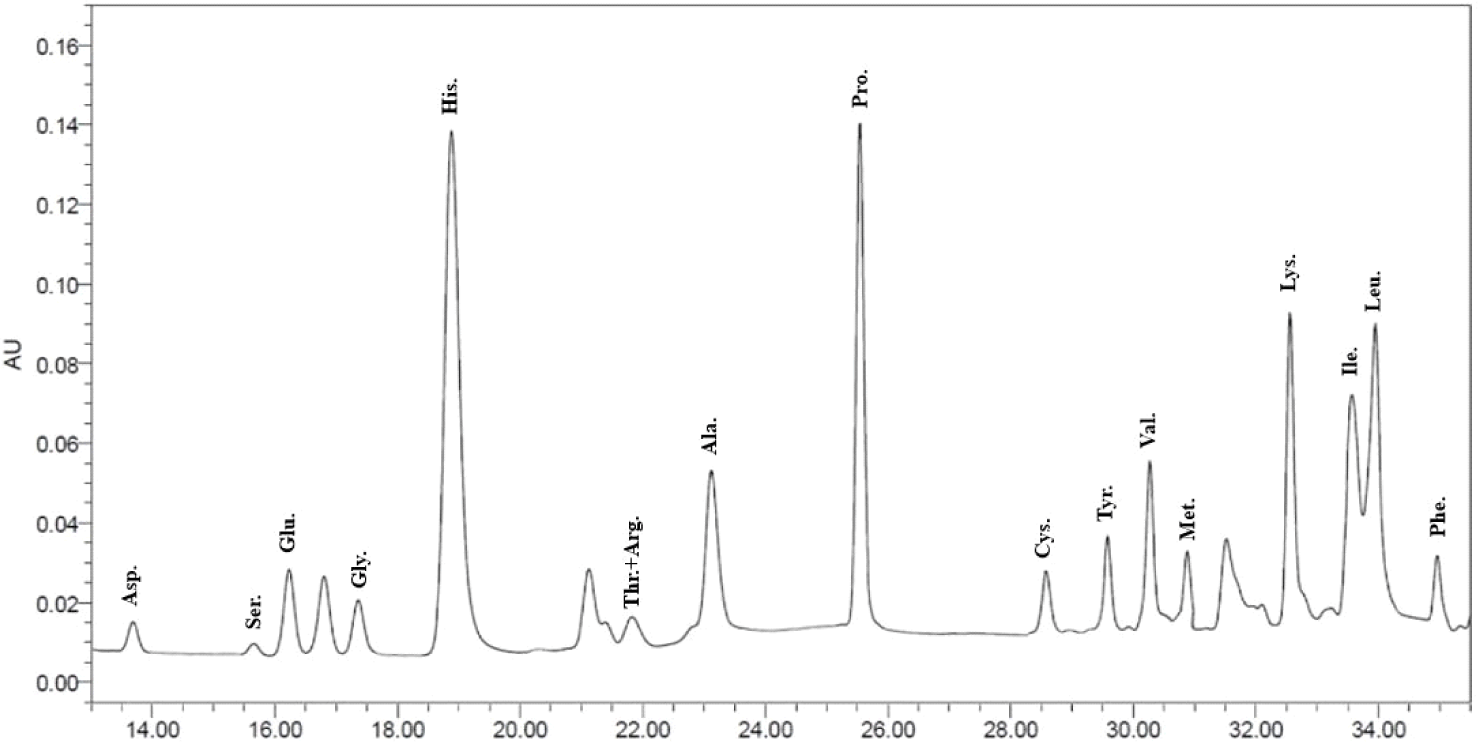
Based on the previous experiment, F5 was finally selected as a sample that can expect excellent functionality. F5 is a fraction of whey-derived protein hydrolyzed using Lactobacillus paracasei DK209. The method used for the analysis used the AccQ-Tag system [10]. As for the amino acid sequence of the fraction, 17 amino acids were detected. As a result of the analysis, the content ratio of isoleucine and valine was high, and the content of serine was low. Isoleucine and valine were notable results because they are essential amino acids that can be obtained only by the influx of external nutrients (Fig. 5).
Discussion
Lactobacillus paracasei DK209 was isolated from kimchi. A fraction of whey-derived protein hydrolyzate was obtained for 20 hours using a Hiprep 16/60 Sephacryl S-100 HR column. A variety of chromatography methods have been proposed to separate each fraction of the peptide. Since this study aims to acquire specific functional peptides, a column separated by molecular weight was used to separate fractions.
In addition, antioxidant activity was verified for each of the five fractions obtained. ABTS, DPPH and Hydroxyl radical scavenging assay were performed for the verification of antioxidant activity.
In each whey protein-derived peptide fraction, F5 showed a similar level of NO production inhibitory activity to that of casein phosphopeptide. There was a study showing the results [18]. Results for inflammatory cytokine inhibitory activity showed that F5 exhibited high inhibitory activity. These points indicate that the functionality of specific peptides can enhance immune capacity [19].
MTT experiments on whey-derived peptides showed a sample associated with the inhibition of NO production. A comparison of the hydrolyzed whey-derived protein with a sample prior to hydrolysis shows the results. As a result of checking the amino acid content ratio of the peptide fractions obtained using the AccQ-Tag system, 17 types of amino acids were identified. Among the amino acid contents, the contents of Ile, Lys, and Val were found to be high. The codons for isoleucine are AUU, AUC, and AUA. It is classified as a hydrophobic amino acid because of the hydrogen-carbon chain it contains. It is a bipolar amino acid and a branched-chain amino acid. Lys is also an essential amino acid and has the characteristic of being a hydrophilic amino acid. Val is also an essential amino acid and is used as an ingredient in muscle-building and protein supplements. And Ile and Val are branched-chain amino acids that continuously supply metabolic energy when the muscle exerts its athletic ability. And it is characterized by resistance to trauma and infection. And the amino acids Lys, Ile, and Cys, which showed varying contents, are known to function as metabolic regulators when performing glucose metabolism in the body and have been shown to affect body weight change [11].
Conclusion
As a result of hydrolyzing whey-derived protein peptides with LAB that confirmed the functionality, the content of free amino acids, which is a nutritional function, was increased. These properties show their applicability to the production of functional products utilizing dietary supplements and milk. These fractions represent efficiency for the mass production of biological peptides. To achieve this point, a system that can uniformly control the physiological properties of LAB is required. Due to the biological characteristics of LAB, the characteristics may change depending on the culture conditions and environment, which may affect certain qualities. If these conditions can be controlled, it can be an efficient method for producing uniform-quality whey-derived peptides.
