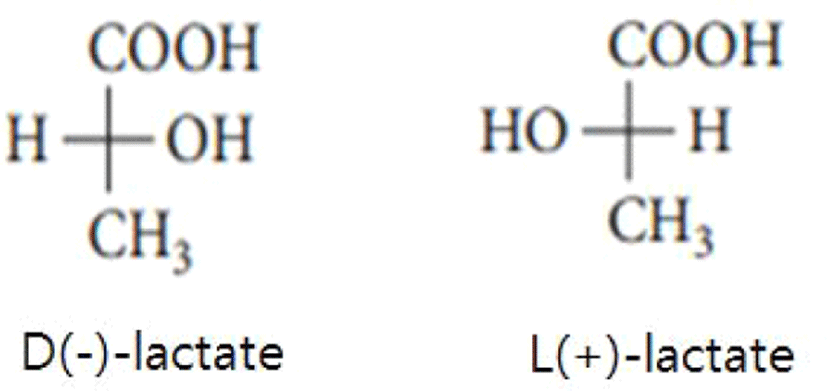Introduction
In general, lactic acid bacteria are recognized as a significant material that continues to arouse great interest because it is widely applied and used in cosmetics, food, pharmaceuticals, textile industries, and so on [1]. Additionally, the three basic roles of lactic acid are to act as a natural preservative in fermented milk products, to ensure that fermented products are manufactured biologically safely, and to help the milk components to be easily digested [2]. Lactic acid could be manufactured in high volume by lactic acid bacteria through homofermentative way such as Embden-Meyerhof pathway, or through heterofermentative way such as phosphogluconate and phosphoketolase pathway [1,3], Lactic acid bacteria could make either one or the two forms of lactate (or lactic acid) [1–4]. In other words, it is already widely known that lactic acid existed in the type of two enantiomers such as D(–)-lactic acid and L(+)-lactic acid (Fig. 1) [2,4]. Lactic acid could remain as a conjugated base lactate [for example, L(+)- or D(–)-lactate] in physiological pH 7.4 [4]. However, the incidence of the conjugated base does not have any effect on the chirality retained in the basic anion [4].
Furthermore, depending on the characteristics of lactic acid bacteria, L(+)-lactate, D(–)-lactate, racemic DL-lactate, or a combination thereof may be produced [5]. To summarize the types of lactate produced by various lactic acid bacteria, Aerococcus, Carnobacterium, Enterococcus, Lactococcus, and Streptococcus produced L(+)-lactate, Leuconostoc and Oenococcus produced D(–)-lactate, Pediococcus produced L(+)-lactate and DL-lactate, Weissella produced D(–)-lactate and DL-lactate, and Lactobaciilus produced L(+)-lactate, D(–)-lactate and DL-lactate [5–7]. Also it was known that fungi such as Phytophthora undulate, Phythium debaryanum, andSapromyces elongatus and bacteria such as Escherichia coli,Fusobacterium nucleatum andPseudomonas aeruginosa could convert D(–)-lactate to pyruvate by reducing NAD+ to NADH under the reaction of the enzyme D(–)-lactate dehydrogenase (cytochrome, EC 1.1.2.4) [4].
The predominant type of lactate generally found in human’s blood was L(+)-lactate, which was come from pyruvate through the activity of L-lactic dehydrogenase [8]. The metabolization of D(–)-lactate in humans progresses at a rate about 30% slower than that of L(+)-lactate [6,8]. The human daily production of lactic acid was approximately 20–25 nmol/kg based on body [9]. The body had the plentiful ability to handle an abnormally high L(+)-lactate concentration but only limited ability to handle an increased concentration of the D(–)-lactate [10]. Under the circumstances, D(–)-lactate could be stored in blood and then result in D(–)-lactic acidosis [9]. A disease with both neurological symptoms and severe metabolic acidosis is known as short bowel syndrome [11]. In 1979, Oh et al. [11] reported a case report after first discovering D(–)-lactic acidosis in a patient with short bowel syndrome. In general, treatments that help relieve short bowel syndrome include a low-carbohydrate diet, bicarbonate therapy, rehydration, oral antibiotics, and so on [12]. Especially, in markedly elevated plasma D(–)-lactate and normal L(+)-lactate conditions, the patient would suffer from the anionic gap acidosis [13]. As of 2018, ninety eight patients suffered from the D-lactic acidosis syndrome (over 3 mM of plasma D(–)-lactate concentration, high anion gap acidosis and neurological symptoms) had been reported, almost all showed the severe problem of short bowel syndrome [13].
Therefore, since most probiotics are composed of lactic acid bacteria, it is considered that a study on the ratio of D(–)-lactate and L(+)-lactate produced by lactic acid bacteria is necessary. Consequently, the aim of this study was to carried out to estimate the ratio of D(–)-lactate and L(+)-lactate of various lactic acid bacteria used as probiotics by enzymatic method.
Materials and Methods
All lactic acid bacteria were obtained from American Type Culture Collection (ATCC, USA), Korean Culture Center of Microorganisms (KCCM, Korea), Korean Collection for Type Cultures (KCTC, Korea), and Samik Dairy & Food (Korea). Fifteen types of lactic acid bacteria used and analyzed in this study consisted of six lactic acid bacteria belonging to Bifidobacterium genus, five lactic acid bacteria belonging to Lactobacillus genus, three lactic acid bacteria belonging to Leuconostoc genus, and one lactic acid bacteria belonging to Pediococcus genus. A list of all strains with their origin is collected in Table 1.
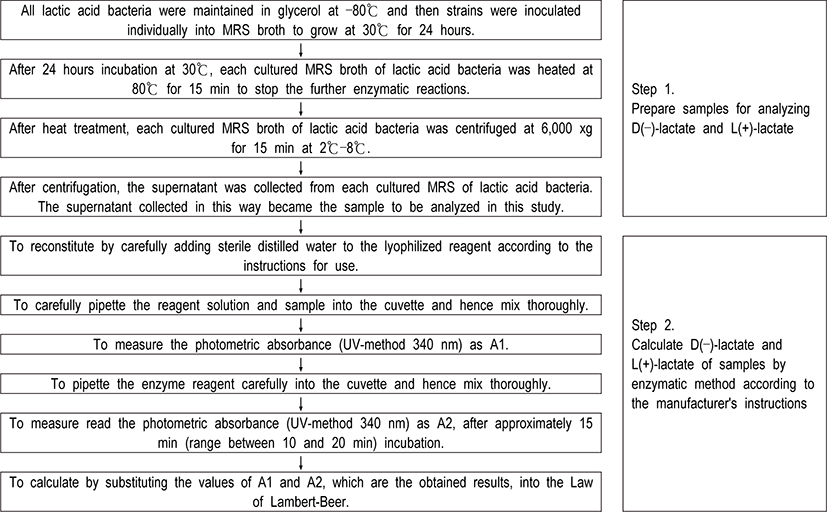
In order to obtain the exact content of D(–)-lactate and L(+)-lactate produced by fifteen various lactic acid bacteria, samples were prepared by processing in the same way as follows. All strains were maintained in glycerol at –80°C and then strains were inoculated individually into MRS broth (Difco, USA) to grow at 30°C for 24 hours. After 24 hours incubation, cultured media were heated at 80°C for 15 min to stop the enzymatic reactions and were centrifuged at 6,000 xg for 15 min. The supernatant as sample was collected and then the calculation of D(–)-lactate and L(+)-lactate by enzymatic method was followed according to the manufacturer’s instructions (Roche enzymatic test kits, Germany) (Fig. 2). For UV measurement in this study, a UV-1700 Spectrophotometer (Shimadzu, Japan) was used.
Repeat experiments on fifteen types of lactic acid bacteria analyzed in this study were conducted at least three times, and hence all data obtained in this study were analyzed to obtain statistically significant differences using the Statistical Program (GraphPad Software, USA).
Results and Disscussion
In this study, the ratio of D(–)-lactate to total lactate [D(–)-lactate + L(+)-lactate] of 15 different lactic acid bacteria were investigated by the enzymatic method. The specific isomer formed was dependent on the species of lactic acid bacteria (Table 1).
In the six lactic acid bacteria belonging to the genus Bifidobacterium, the ratio of D(–)-lactate to total lactate was as follows, showing 34.90% in B. adolescent KCCM 3216, 26.13% in Bifidobacterium bifidum BB12, 27.68% in B. bifidum BB46, 45.76% in B. infantis B1710, 44.60% in B. infantis KCTC 3247, and 28.98% in B. longum KCTC 3215, respectively (Table 1). The range of D(–)-lactate in lactic acid bacteria of the genus Bifidobacterium analyzed in this study was 28.98%–45.76%. And the range of D(–)-lactate and L(+)-lactate of the genus Bifidobacterium analyzed in this study was 0.31–13.9 mM and 0.76–39.3 mM, respectively (Table 1).
The ratio of D(–)-lactate to total lactate was as follows, showing 61.02% in Lactobacillus acidophilus KCCM 32820, 47.37% in Levilactobacillus brevis ATCC 13648, 55.67% in L. bulgaricus LB-12, 41.18% in L. confuses KCCM 40015, and 46.61% in Lactiplantibacillus plantarum ATCC 14917 in the 5 lactic acid bacteria belonging to the genus Lactobacillus, respectively (Table 1). The range of D(–)-lactate in lactic acid bacteria of the genus Lactobacillus analyzed in this study was 41.18%–61.02%. And the range of D(–)-lactate and L(+)-lactate of the genus Lactobacillus analyzed in this study was 1.08–11.7 mM and 0.69–13.0 mM, respectively (Table 1).
In the ratio of D(–)-lactate to total lactate of lactic acid bacteria belonging to the genus of Leuconostoc, L. cremoris KCCM 35467 was 38.60%, L. dextrancum KCCM 35046 was 29.85%, and L. mesenteroides KCCM 11324 was 42.36%, respectively (Table 1). The range of D(–)-lactate in lactic acid bacteria of the genus Leuconostoc analyzed in this study was 29.85%–42.36%. And the range of D(–)-lactate and L(+)-lactate of the genus Leuconostoc analyzed in this study was 0.72-20.3 mM and 0.98–32.3 mM, respectively (Table 1).
Pediococcus acidilacti KCCM 11747 showed 45.71% of the ratio of D(–)-lactate to total lactate (Table 1). And D(–)-lactate and L(+)-lactate of Pediococcus acidilacti KCCM 11747 analyzed in this study was 33.0 mM and 39.2 mM, respectively (Table 1).
A comparison of the results of this study with similar previous studies conducted by other researchers was as follows.
Alm [2] reported that the content of L(+) and D(–) lactic acid in regular milk, cultured buttermilk, yoghurt, kefir, ropy milk, low fat milk, low fat acidophilus milk, V-medium, acidophilus milk, and bifidus milk was obtained by the enzymatic method. Although L(+)-lactate was the main component in all products, D(–)-lactate was 0%–10% in acidophilus milk and 40% in yoghurt, respectively [2]. Also Alm [2] reported that the main metabolite formed during the fermentation of milk was L(+)-lactate, which was in the same form as lactic acid produced in the human body. In addition, significant amounts of D(–)-lactate could be detected in yogurt [2]. The D(–)-lactate would reduce cell metabolism and then would cause acidosis in ruminants and in humans [2]. Hence, World Health Organization strongly insisted on strictly limiting the intake of D(–)-lactate [2]. The reason for this background was that the metabolic rate of D(–)-lactate was lower than that of L(+)-lactate [6,8]. Furthermore, Pohanka [4] reported that the proportion of D(–)-lactate to total lactate in the blood of three patients suffered from short bowel syndrome was investigated. Specifically, it showed a tendency to increase as age decreased. Namely, the ratio of D(–)-lactate to total lactate in 14-year-old patients was 64.3%, that of 9-year-old patients 86.4%, and that of 5-year-old patients 89.9%, respectively [4]. Also, formula with 80% L(+)-lactate and 20% D(–)-lactate could lead to development of severe acidosis symptoms in one infant [14]. When infant formula containing 0.35 g of lactic acid per 100 g was consumed, the secretion of organic acids in urine including D(–)-lactate tended to increase [8].
Next, Kodama et al. [15] reported of a study that established the capillary electrophoresis method for rapid detection of lactate in various foods. In particular, the reason for using the capillary electrophoresis method is that most of these chromatography methods have not been used to chiroptically separate lactate from fermented foods [15]. The most suitable running conditions for lactate separation using capillary electrophoresis were 90 mM phosphate buffer (pH 6.0) including 240 mM 2-hydroxypropyl-β-cyclodextrin with the effective voltage of –30 KV at 16°C using direct detection at 200 nm under a pressure of 50 mbar during 200 seconds [15].
Also, when the capillary electrophoresis method and the enzymatic method were performed, the ratio of D(–)-lactate to total lactate were 22.8% and 23.3% in yoghurt A, 14.2% and 15.2% in yoghurt B, 7.5% and 6.7% in yoghurt C, 18.6% and 20.3% in wine, 24.7% and 26.8% in sake, 47.5% and 45.4% in beer, and 50.0% and 50.0% in soft drink, respectively [15]. Namely, there was no difference in detection ability between the enzymatic method and the capillary electrophoresis method in measuring the lactate content [15]. And the concentration of D(–)-lactate and L(+)-lactate obtained from enzymatic method was 21.33 mM and 69.60 mM in yoghurt A, 11.11 mM and 61.16 mM in yoghurt B, 4.65 mM and 68.49 mM in yoghurt C, 4.34 mM and 16.87 mM in wine, 1.91 mM and 5.19 mM in sake, 0.49 mM and 0.58 mM in beer, and 0.35 mM and 0.35 mM in soft drink, respectively [15]. Therefore, it is thought that it would be better to improve the reliability of the obtained results if the capillary electrophoresis method and the enzymatic method are used complementary in lactate analysis.
The production of milk and milk beverage could be complicated by D(–)-lactate contamination relying on the kind of bacterial presence [4]. The protective effect of probiotics has recently been demonstrated and its application is directly or indirectly related to the decrease of D(–)-lactate concentration [16]. D-lactate could be hyperproduced by microbiota under specific situation such as short bowel syndrome and jejunoileal bypass surgery further supported that the patient had the meal with high sugar level [17]. D(–)-lactate may be included in food and beverages either produced by biotechnology processes or contaminated by microorganisms and various manners [18]. Other symptoms of D(–)-lactate poisoning included general confusion, dizziness, headache, aggressive behavior, memory loss, and so on [19]. Even though D(–)-lactate was not a highly toxic chemicals affecting severely hazarded to the life of human, it could lead to health problems and complex other pathologies [4].
Recently, the attention in D(–)-lactate is also increasing in the context of increasing interest in human’s health. In this paper, the D(–)-lactate ratio of some lactic acid bacteria among the currently widely used lactic acid bacteria was investigated by the enzymatic method. In the future, there is an urgent need to not only test for D(–)-lactate on various fermented products (for example, Kefir, Koumiss, cheese, yoghurt, Korean- type fermented foods, Asian-type fermented foods, Western-type fermented foods, and so on) with the addition of various lactic acid bacteria recognized as probiotics, but also to study the screen for various lactic acid bacteria (used as probiotics) that produce the level of D(–)-lactate.
Conclusion
There were three different isomeric types of lactic acid such as L(+)-lactate, D(–)-lactate, and DL racemic mixture. These were produced as metabolites through carbohydrate metabolism of various lactic acid bacteria, and their ratio and level would vary depending on the genus and species of lactic acid bacteria. Especially, the rate of metabolism of the D(–)-lactate was considerably lower than that of L(+)-lactate. If large amounts of D(–)-lactate are consumed continuously, D-lactic acidosis may occur in humans and ruminants. Therefore, it is recommended to refrain from consuming products containing high amounts of D(–)-lactate. In this study, the level and ratio of L(+)-lactate and D(–)-lactate in fifteen lactic acid bacteria were quantitatively analyzed by the enzymatic method. The range of D(–)-lactate and L(+)-lactate was 0.31–13.9 mM and 0.76–39.3 mM in the genus Bifidobacterium, 1.08–11.7 mM and 0.69–13.0 mM in the genus Lactobacillus, 0.72–20.3 mM and 0.98–32.3 mM in the genus Leuconostoc, and 33.0 mM and 39.2 mM in Pediococcus acidilacti KCCM 11747, respectively (Table 1). And in the ratio of D(–)-lactate to total lactate, B. adolescent KCCM 3216 was 34.90%, B. bifidum BB12 was 26.13%, B. bifidum BB46 was 27.68%, B. infantis B1710 was 45.76%, B. infantis KCTC 3247 was 44.06%, B. longum KCTC 3215 was 28.98%, L. acidophilus KCCM 32820 was 61.02%, L. brevis ATCC 13648 was 47.37%, L. bulgaricus LB-12 was 55.67%, L. confuses KCCM 40015 was 41.18%, L. plantarum ATCC 14917 was 46.61%, L. cremoris KCCM 35467 was 38.60%, L. dextrancum KCCM 35046 was 29.85%, L. mesenteroides KCCM 11324 was 42.36%, and Pediococcus acidilacti KCCM 11747 was 45.71%, respectively (Table 1). Namely, each lactic acid bacteria analyzed in this study showed various ratio of D(–)-lactate to total lactate. More importantly, there was a big difference in the D(–)-lactate and L(+)-lactate contents actually produced by each of the lactic acid bacteria investigated in this study. As mentioned earlier, additional analysis and consideration of the characteristics of each lactic acid bacteria showing various differences in D(–)-lactate and L(+)-lactate contents are required. In the future, studies on the selection of lactic acid bacteria that have effects on D(–)-lactate pathophysiology as well as the effects of D(–)-lactate produced by lactic acid bacteria on human health should be conducted.