Introduction
Milk is made of milk fat, protein, carbohydrates, water-soluble vitamins, minerals, and water. Among them, whey protein and casein present as protein components. Casein is major component of milk protein and it is widely studied because it has high availability and nutritional importance. It is about 3% in milk and makes up about 80% of all protein in milk and it consists of α, β, γ, κ-casein, molecular weight is about 75,000–375,000 kDa [1].
Casein is a source of exogenous and endogenous amino acids and a precursor of biologically active peptides characterized by immunogenic, antioxidant, antibacterial, antiviral, antihypertensive, antitumor and opioid properties [2].
Also, the enzymatic hydrolysis of casein may act in the body as regulatory components with a hormone activity which may modulate specific physiological functions [3].
In vitro and in vivo enzymatic proteolysis of casein proteins produce bioactive peptides [4], which have specific biological functions throughout their ability to affect cellular function [5].
Among them, antioxidant activities of milk protein hydrolysates and milk-derived peptides obtained by fermentation have recently investigated [6]. The antioxidant activities of the protein hydrolysates seem to be inherent to the characteristic amino acid sequences of peptides derived, depending on the specificity of protein enzyme. Results of their studies suggested that the hydrolysates from milk proteins could be used as natural antioxidant materials of functional foods and in preventing oxidation reaction in food processing. In addition, casein trypsin digests showed inhibitory properties in the oxidation of linoleate by lipoxygenase, peroxyl radicals and 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical [7]. Casein and casein peptides can bind iron [8], thus making them potential antioxidants in food systems. Concentrated casein phosphopeptide (CPP) and casein hydrolysates have antioxidant activities in corn oil-in-water emulsions at pH 7.0 and 3.0 [9]. In addition, in relation to the antibacterial activity of the casein hydrolysates, the antibacterial activity has been reported more than 8 times higher than the original protein [10]. A structural group of casein antibacterial peptides discovered and the association between its structure and antibacterial activity found [11].
In this regard, the aims of this study are development of specific peptides that have physiological properties of hydrolyzed casein and have similar effects to CPP, a material that is commercially available.
Materials and Methods
Commercial casein (95% protein) was obtained from Meggle food Ingredients (Wasserburg, Germany).
10% casein solution prepared by mixing 10% casein and 90% 0.1 M Potassium phosphate buffer (pH 7.4). After a sterilization for 10 minutes at 90°C of casein solution and it adjusted to pH 7.0, the optimum active pH of the enzyme, using a 1 N NaOH (Sigma, USA).
Commercial enzymes used as Alcalase® 2.4 L FG (Protease from Bacillus licheniformis, 5 U/g), Neutrase® 0.8 L (Protease from Bacillus amyloliquefaciens, 0.8 U/g), Protamex® (Protease from Bacillus sp., 1.5 U/g) and Flavourzyme® (Protease from Aspergillus oryzae, 500 U/g). All commercial enzymes purchased from NovozymesTM (Denmark).
The enzymes added at a ratio of 1:200 (w/v) based on the substrate, and then shaken at 120 rpm in a shaking water bath at 50°C. Samples taken at 30 minute intervals to inactivate the enzymes at 90°C for 10 minutes and proceeded to 240 minutes. After a cooling at 20°C, the supernatant collected by centrifugation for 20 minutes under conditions of 4,000 g and 4°C. The supernatant filtered using PVDF 0.22 Syringe membrane filter (Futecs, Korea). Samples stored at −20°C and used for the experiment.
The hydrolysis degree of casein hydrolysates measured using the Lowry method [12]. Lowry solution A and B prepared for the degree of hydrolysis (DH). To prepare solution A, 1% cupric sulfate (Sigma) and 2% sodium potassium tartrate (Sigma) were mixed at a 1:1 ratio (v/v), and 1.0 mL of the mixed solution and 50 mL of 2% Na2CO3 (DaeJung Chemical & Metals, Korea) mixed. Solution B used to prepare a 1 N Folin-Ciocalteau reagent (Sigma).
First, 1.0 mL of casein hydrolysates and the same amount of 20% TCA (Duksan Chemical, Korea) mixed and it maintained for 1 hour to precipitate 20% TCA soluble protein. After that, it centrifuged at 18,000 ×g for 20 minutes. The supernatant and redissolved residue removed in 5.0 mL of 0.1 N NaOH (Sigma).
Thereafter, 1.0 mL of casein hydrolysates and 5.0 mL of solution A mixed and reacted at room temperature for 10 minutes Then, 0.5 mL of solution B added and the contents immediately mixed using a vortex mixer. After reacting for 30 minutes, absorbance measured at 600 nm. Equivalent amounts of protein calculated from standard curve prepared using bovine serum albumin (Sigma). Absorbance of bovine serum albumin measured in the range from 20 to 200 μg/mL.
The amount of TCA soluble protein measured and calculated by the following equation.
SDS-PAGE performed to obtain a profile of casein hydrolysates. The molecular weight of the casein hydrolysates measured using Bio-rad devices including gel electrophoresis Mini-protein tetra electrophoresis system [13].
12% Acrylamide gel (Bio-Rad Lab, USA) prepared to analyze a casein hydrolysates protein profile. Protein bands stained with Coomassie brilliant blue R-250 (Bio-Rad Lab). The molar mass standard used Precision plus protein dual xtra standards (Bio-Rad Lab).
In order to obtain peptide fractions from casein hydrolysates, gel filtration using a preparative chromatography system performed (Waters Corp, USA).
Peptides that identified in SDS-PAGE separated throughout a preparative scale for further study. The condition of preparative liquid chromatography system is shown in Table 1. The preparative pump (Waters Corp) and Preparative liquid chromatography W600 (Waters Corp) were used with a Dual λ Absorbance detector W2487 (Waters Corp) and W717 Autosampler (Waters Corp).
The casein hydrolysates dissolved in 5 mM sodium phosphate buffer with 0.15 M NaCl (Sigma), pH 7.0. It filtered using a PVDF 0.45 μm sterile syringe membrane filter (Futecs). Next, the casein hydrolysates loaded 3.0 mL on the Hiprep 16/60 Sephacryl S-100 HR column (GE Healthcare Life Sciences, USA) and eluted at flow rate of 1.0 mL/min. A detector was set at 280 nm.
All fractions that obtained during gel filtration using Hiprep 16/60 Sephacryl S-100 HR column throughout a preparative chromatography system collected and fractions stored at −20°C.
For ABTS radical scavenging activity, ABTS powder (Sigma) mixed to ethanol and 7 mM ABTS was prepared. Thereafter, ABTS mixed with 2.45 mM potassium persulfate (Sigma) in a 1:1 (v/v) ratio to prepare an ABTS solution. This solution stored in dark room for 12–16 hours. The ABTS stock solution diluted in ethanol to an absorbance of 0.70±0.02 at 734 nm.
After mixing 190 uL of the diluted ABTS solution to 10 uL of the fraction sample, and the absorbance taken 3 min later.
The results expressed in units of Trolox (Sigma) equivalent antioxidant activity in units of mM/L.
The 0.2 mM DPPH solution prepared by mixing 2,2-diphenyl-1-picrylhydrazyl,2,4,6-tripyridyl-s-triazine (Sigma) and ethanol (Samchun, Korea).
Thereafter, DPPH solution and fraction sample mixed in a 1:1 ratio (v/v) and reacted in a dark room for 30 minutes. The absorbance measured at 570 nm, and results expressed in units of ascorbic acid (Sigma) equivalent capacity in units of mM. All protein concentration of fraction samples is 200 μg/mL.
The FRAP solution for use in the experiment was prepared. The 300 mM acetate buffer prepared by mixing Sodium acetate trihydrate (Sigma) and acetic acid (Samchun). The 10 mM TPTZ prepared by dissolving 2,4,6-tri(2-pyridyl)-1,3,5-triazine (Sigma) in 40 mM HCl (Samchun), and the 20 mM Iron(III) chloride hexahydrate solution prepared by dissolving Iron(III) chloride hexahydrate 97% A.C.S reagent (Sigma) in ethanol (Samchun). The above three solutions were mixed in a ratio of 10:1:1 (v/v).
Also, 1.5 mL of the FRAP solution pre-warmed to 37°C mixed with 50 μL of the casein hydrolysate fraction samples and standards. The mixture vortexed and reacted in a dark room at room temperature. The absorbance measured at 593 nm.
The results expressed in units of Ascorbic acid (Sigma) equivalent capacity in units of mM. All protein concentration of casein hydrolysate fraction samples were 200 μg/mL.
The 0.75 mM 1,10-phenanthroline (Sigma), phosphate buffer (pH 7.0), 2.5 mM FeSO4 (Sigma) and casein hydrolysate fraction samples mixed in a ratio of 1:1:1:1 (v/v). The 20 mM Hydrogen peroxide (Junsei Chemical, Japan), the reaction reagent finally mixed in the same ratio (v/v). The reaction carried out in a water bath at 37°C for 90 minutes. The absorbance measured at 593 nm.
The results expressed in units of Ascorbic acid (Sigma) equivalent capacity in units of mM. All protein concentration of casein hydrolysate fraction samples were 200 μg/mL.
Antibacterial activity evaluated using a paper disc method [14]. Pathogenic bacteria Escherichia coli KCTC1682, Salmonella enterica KCTC2054, Bacillus cereus KCTC362A and Staphylococcus aureus KCTC3881 obtained from the Korean Collection for Type Cultures (KCTC) and it dispensed on Mueller hinton agar (Difco™, USA). One hundred microliter of the pathogenic bacteria culture (103–104 CFU/mL) uniformly distributed by pouring 30 mL of pre-sterilized Mueller hinton agar and allowed for solidification.
A paper disc method (Advantec, Japan), impregnated with the compound, placed on the surface of the Mueller hinton agar. The 100 μL of casein hydrolysate fractions dispensed into each paper disc and incubated in the incubator for 37°C. After incubation for 24 hours, the diameter (mm) of the inhibition clear zone around the well measured and compared to each other.
Results
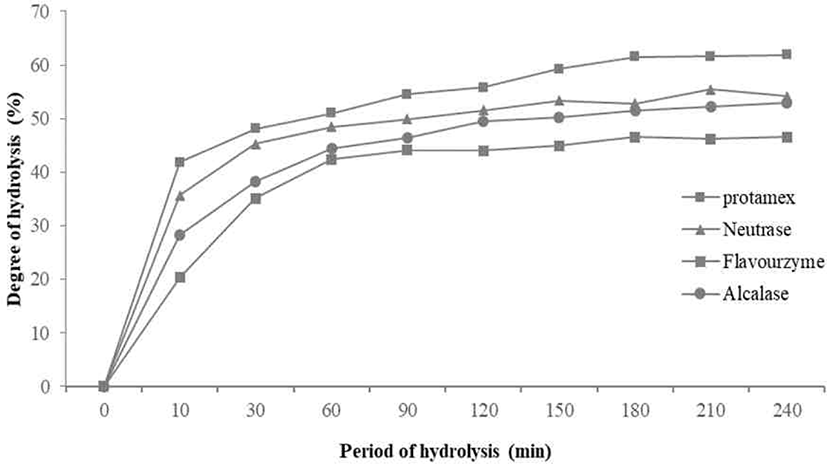
The degree of casein hydrolysis defined as the percentage of the total number of peptide bonds in a protein that cleaved during hydrolysis [15]. The DHs of the four enzymes for casein tended to increase in a similar pattern. There was no significant difference from 150 minutes after increasing from 120 minutes to 150 minutes. Among them, the casein hydrolyzed by Protamex® measured with highest DHs, and the DHs of the casein hydrolyzed by Neutrase® and Flavourzyme® were similar (Fig. 1).
SDS-PAGE performed to confirm the hydrolysis characteristics of the casein. Protein bands with a molecular weight range of 2 to 250 kDa identified (Fig. 2). αs2-casein, αs1-casein, β-casein and κ-casein, etc., the main components of casein, found from bands between 25 and 37 kDa. The numbers of band in the region with lower molecular weight increase gradually with enzymatic hydrolyzation. It showed the peptides with higher molecular weight broke up during enzymatic hydrolysis.
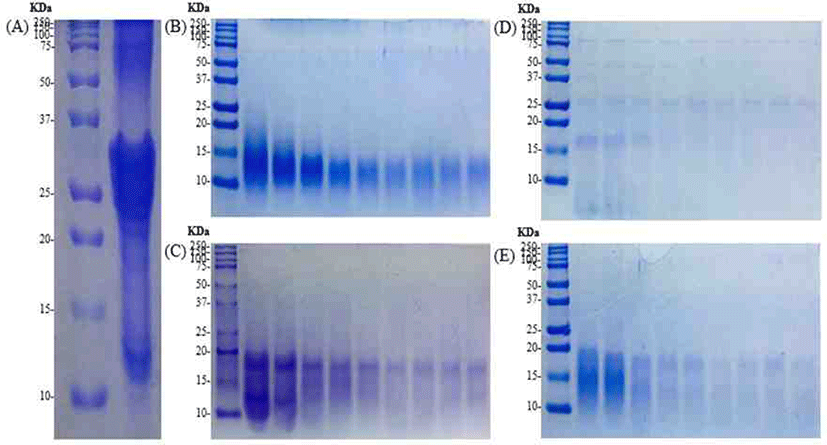
Except for Flavourzyme®, in the case of Alcalase®, Neutrase® and Protamex® hydrolysates, αs2-casein, αs1-casein, β-casein and κ-casein hydrolyzed, and it confirmed that bands formed between 10 and 20 kDa. In the case of Alcalase® hydrolysates, fewer bands observed compared to other hydrolysates.
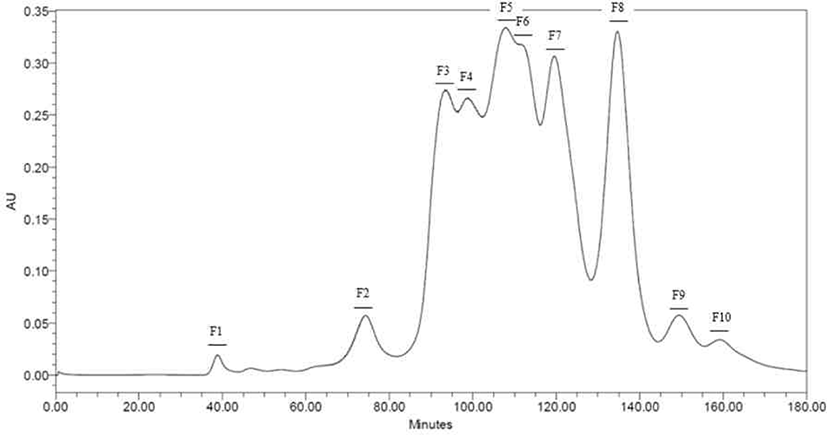
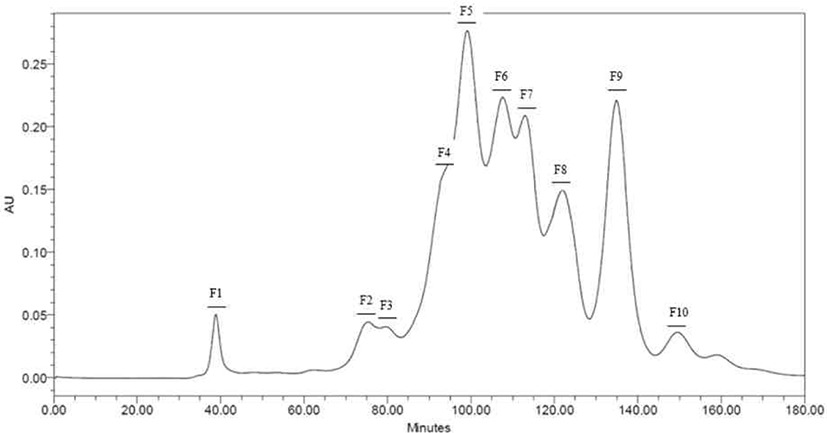
Peptides identified in SDS-PAGE and separated throughout the preparative scale for further study. Neutrase® and Protamex® selected with base on results of SDS-PAGE and DHs. Peptide fractions of casein hydrolysates isolated using Hiprep 16/60 Sephacryl S-100 HR column throughout a preparative liquid chromatography system. Each fraction separated by molecular weight. Total of ten peptide fractions obtained(Fig. 3 and 4). Each fraction compared with original casein hydrolysates via SDS-PAGE. As a result, it proved which the fraction by molecular weight (Data not shown).
The ABTS has a property of becoming transparent when it encounters a substance having antioxidant activity.
According to results (Fig. 5), F3, F4, F5, F6, and F7 in the hydrolysate fractions using Neutrase® showed similar level of activity as CPP. Except for F1, F2, F8, F9, and F10, other fractions showed higher ABTS radical scavenging activities than non-hydrolyzed casein (NHC), of which F3 had highest activity.
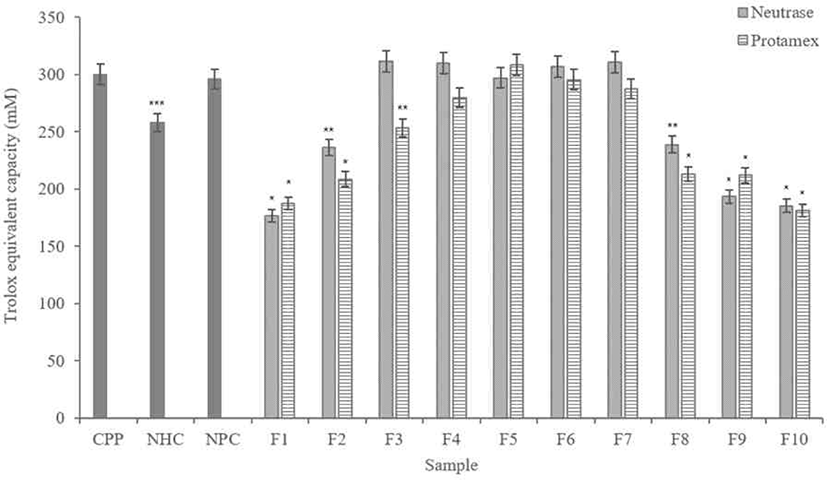
In the Protamex® hydrolysate fractions, F5 and F6 showed similar scavenging activity with the CPP. The F5 showed the highest scavenging activity than all fractions including the Neutrase® hydrolysate fractions. It is also higher than CPP, NHC and non-purified casein (NPC).
The DPPH free radical is a stable free radical, which has been widely accepted as a tool for estimating the free-radical scavenging activities of antioxidants.
In this study, antioxidant activities evaluated by compared to value DPPH scavenging activity. Based on the standard curve of Ascorbic acid (Vitamin C), DPPH scavenging activity values statistically analyzed.
According to results (Fig. 6), F4 and F7 in the Neutrase® hydrolysate fractions showed DPPH radical scavenging ability similar with the CPP. Among them, F4, F5, and F6 showed higher scavenging ability than NHC and NPC.
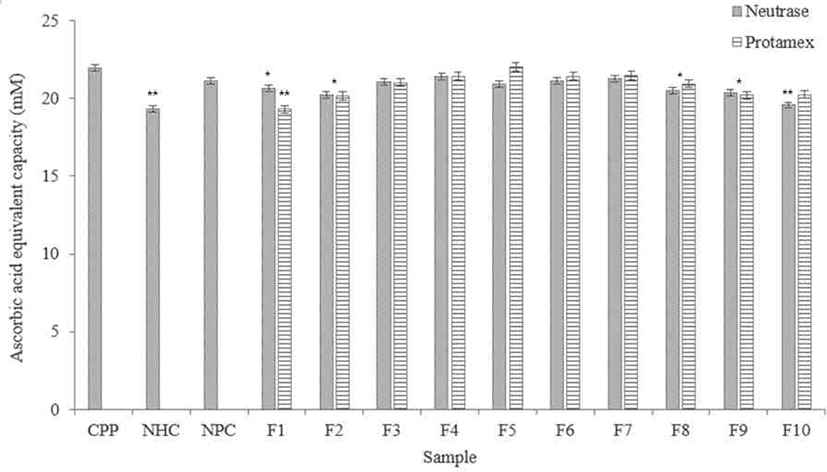
The F5 in the Protamex® hydrolysate fractions showed excellent DPPH radical scavenging activity, similar with the CPP. F4, F6, and F7 in the fractions not significantly difference with the CPP. All fractions showed higher DPPH radical scavenging activity than NHC.
FRAP assay is direct way to measure antioxidant activities. It is based on activities of antioxidant to reduce Fe3+ to Fe2+ in the presence of TPTZ forming an intense blue Fe2+-TPTZ complex with an absorption at 593 nm. The reducing potentials of antioxidants are associated with their electron donating abilities to break the free radical chain reaction [16].
According to results (Fig. 7), FRAP measurements showed that Neutrase® hydrolysate fractions showed similar data in iron reducing capacity. F3 and F5 showed the most similar iron reduction capacity with the CPP compared to other fractions. F2, F9, and F10 showed lower activity than NHC.
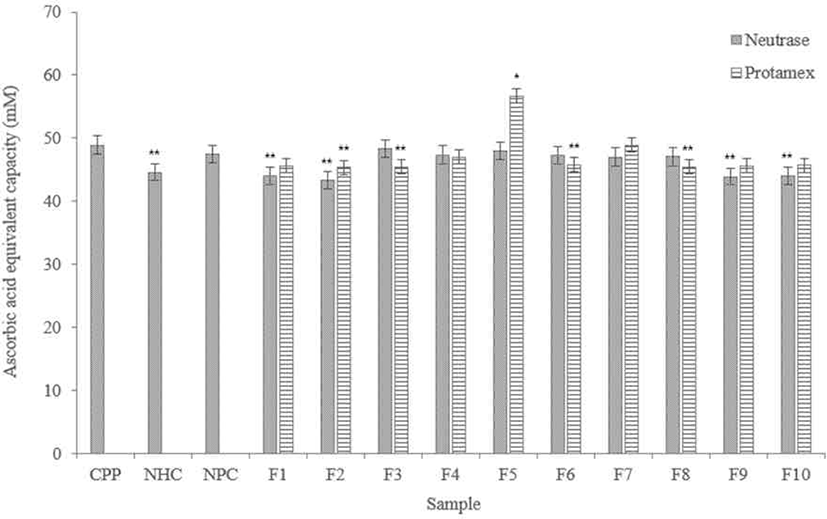
Among the Protamex® hydrolysate fractions, F5 showed significantly higher iron reducing capacity, and it is higher than the CPP. In addition, all fractions have higher activities than NHC, and F7 showed a similar level with the CPP.
Hydroxyl radical is the most reactive oxygen species produced by the reduction of three electrons from oxygen.
According to results (Fig. 8), Neutrase® hydrolysate fractions showed high radical scavenging activities except for F8, and it showed similar activities with NHC and NPC. Among them, F7 showed the highest scavenging activity, and F3 and F4 showed similar scavenging activity with the CPP.
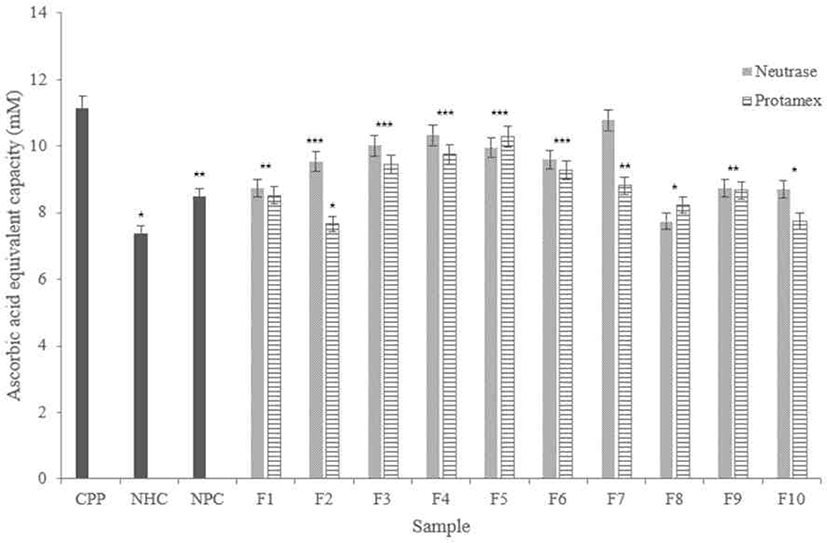
In the Protamex® hydrolysate fractions, F5 showed similar scavenging activity with the CPP. F3, F4, and F6 showed higher radical scavenging ability than other fractions. In addition, all fractions showed higher scavenging capacity more than NHC.
The agar diffusion assay (or inhibition zone assay) is a common method used to test the antibacterial activity of commonly used food, peptides and hydrolysates [17]. The ability of antibacterial activities for Neutrase® hydrolysate fractions measured in terms of zone of inhibition (cm) and values depicted in Table 2.
| Sample | Degree of inhibition of peptide1) | S. enterica | ||
|---|---|---|---|---|
| B. cereus | E. coli | S. aureus | ||
| PC | + | |||
| CPP | + | ++ | ++ | + |
| F3 | ++ | +++ | + | ++ |
| F4 | + | ++ | + | ++ |
| F5 | ++ | ++ | ++ | ++ |
| F6 | ++ | ++ | ++ | ++ |
| F7 | ++ | +++ | ++ | ++ |
According to results (Fig. 9), In the Neutrase® hydrolysate fractions, the highest inhibitory fraction for all pathogens was F7. The F7 showed higher activity more than both positive control (PC) and CPP. The antibacterial activities of other fractions are similar, but it significantly low in the case of F4. All fractions have highest inhibitory for Escherichia coli, and it is higher than PC and CPP.
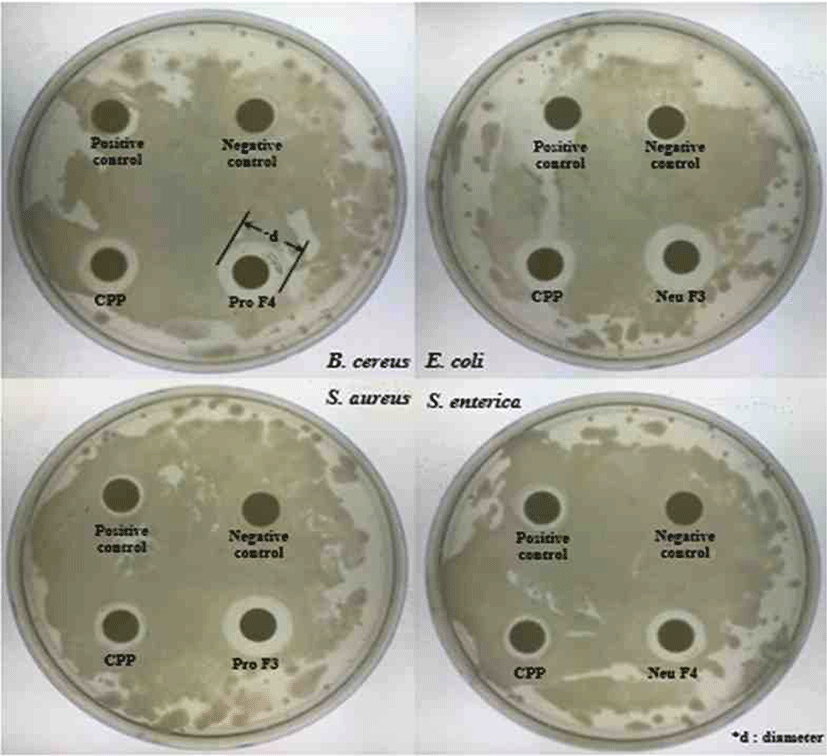
The abilities of Protamex® hydrolysate fractions measured in terms of zone of inhibition (cm) and values depicted in Table 3. In the case of the Protamex® hydrolysate fractions, the inhibitory activity of F3 was the best for all pathogens. All fractions showed good inhibitory activities for E. coli, and the inhibitory activity for Salmonella enterica was almost similar except for F5.
| Sample | Degree of inhibition of fractions1) | S. enterica | ||
|---|---|---|---|---|
| B. cereus | E. coli | S. aureus | ||
| PC | + | |||
| CPP | + | ++ | ++ | + |
| F3 | ++ | +++ | +++ | ++ |
| F4 | +++ | ++ | ++ | ++ |
| F5 | ++ | +++ | ++ | + |
| F6 | ++ | ++ | + | ++ |
| F7 | ++ | ++ | ++ | ++ |
Discussion
In this study, proteolytic enzymes used to focus on the function of specific peptides derived from the hydrolysis of casein.
As a result, the DHs of the four enzymes tended to increase in a similar pattern. There was no significant difference after increasing from 120 minutes to 150 minutes. DH of Protamex® found to increase most appropriately. All enzymatic hydrolysates maintained from 30 to 40 minutes and gradually increased and maintained after 50–60 minutes. In this study, casein contained 95% of protein, and the retention time of hydrolysis was 10 minutes.
The SDS-PAGE performed to confirm the hydrolysis characteristics according to the enzyme. αs2-casein, αs1-casein, β-casein and κ-casein, etc., the main components of casein, found to bands between 25 and 37 kDa. In casein, hydrolyzed with trypsin, casein phosphopeptide (CPP) material obtained. αs2-casein, αs1-casein, β-casein and κ-casein hydrolyzed to identify bands up to 20 kDa. This result is similar with SDS-PAGE results of the enzyme hydrolysates except for Flavourzyme®. This is the basis for confirming the potential to obtain a peptide material of similar molecular weight size with the CPP.
Peptides identified in SDS-PAGE and it separated on preparative scale for further study. In this study, fractions separated by molecular weight throughout a Hiprep 16/60 Sephacryl S-100 HR column. In another study, FPLC used either size exclusion or ion exchange chromatography [18]. Chromatographic methods for peptide separation are very diverse, but the purpose of this study is obtainments of peptides similar with CPP, so fractions separated using a column by molecular weight. The fractions confirmed to be consistent with the hydrolysate molecular weight confirmed by SDS-PAGE (Data not shown).
After that, the antioxidant activities of each enzymatic hydrolysates fraction verified. According to results of ABTS, NF3, NF4, NF5, NF6, and NF7 showed similar level of activities with CPP. Also, PF5 and PF6 showed similar scavenging activity with the CPP. Comparing to results of Neutrase® hydrolysate fractions and Protamex® hydrolysates fraction, the hydrolysis degree test results generally excellent in Protamex® hydrolysates fraction. As a result of DPPH, activities of NF4 and NF7 are similar with CPP, and activities of PF4 and PF6 are similar with CPP. Similarly in the FRAP and Hydroxyl radical scavenging assay results, it confirmed that certain fractions have similar activities with the CPP. According to above results of antioxidant activities, it can be seen that the antioxidant activity and iron reducing ability of Neutrase® hydrolysates fraction is significantly higher than Protamex® hydrolysates fraction. In the previous experiments, Protamex® found to have higher casein hydrolysis than Neutrase®. However, antioxidant activity confirmed that Neutrase® measured higher regardless of this. For example, in previous studies, egg protein that hydrolyzed with trypsin and alcalase showed the highest DHs, but the highest antioxidant activities of samples that hydrolyzed with alcalase [19]. It showed that there is no correlation between hydrolysis and antioxidant activity. This is not only because of differences in substrates but also because of the different cleavage sites for each enzyme. Since the type of amino acids located at the N- and C-terminal of the cleavage site is different, it is reported that the antioxidant activity is different.
As a result of the antibacterial activity test, several fractions showed high antibacterial activity against four pathogens. Indeed, previous studies have shown that αs2-casein and αs1-casein have antibacterial activity from pathogens such as E. coli and S. aureus [20]. In addition, it has reported that Lactoferrin obtained from Bovine lactoferrin present in milk had 8 times more antibacterial activity than the original protein. Based on this, the antibacterial activity of the peptide fraction derived from casein could be demonstrated. Therefore, these protein fractions must be obtained and produced active peptides from them to use as dietary supplements and milk based nutraceuticals. This study identified the potential of new biologically active peptides derived from milk proteins that affect the food and healthcare industry.
