Introduction
The skin of women in pregnancy and during breast-feeding usually loses skin elasticity and can not easily recover to beautiful skin as in youth. In addition to skin health conditions, the women in pregnancy and breast-feeding may not return to their prepregnant weight or body composition by the end of the lactation period (Winkvist and Rasmussen, 1999). The intake of nutrition supplement foods for the development of fetal in pregnancy, and the providing of water enough for breast-feeding are very important. However, lactating mothers want to be recovered their figures and skin, and facial conditions as before (Rhu et al., 2017). The most interesting concern focused on the nutrition, but the most concern for themselves are on restoring of body figure and recovering of skin elasticity (Rhu et al., 2017). Recently, a different type of skin care foods targeted for pregnant women such as tiger nut Galsu drink (Jung et al., 2018), the cookie composed of three kinds of beans; lentil, black bean, chickpea, with brewers’ yeast (Lee et al., 2018). Sea tangle cookie for improving constipation (Choung et al., 2017) and ginger extract candy for calming of morning sickness are also known (Kim et al., 2018). Tiger nut “Galsu” drink for supplying the mothers with enough water (Jung et al., 2018) is under a provisional patent application in Korea.
Avocado has used as fresh food or vegetable oil and raw material of the cosmetics. The avocado (Persea americana), a native fruit of Central and South America, is used as foods from ancient countries such as Aztecs, Incas and Mayans, but it had also used to pasted avocado on face skin like a facial mask (James Bennett, 2018). In the 1920s, the American Pomological Society and the USDA began to use marketable name ‘avocado’ (Popenoe, 1934). Avocado has benefits to moisturize skin by penetrating the oil deep into the skin, helping to soften and hydrate dry and flaky patches. Avocado facial mask is widely using as folk remedies to reduce wrinkles and signs of aging and getting soothe the dry facial skin, and be nourished by slough away dead skin cells and restore a more youthful glow to face (Oliver, 2013).
Many commercial cosmetic products using avocado oil as ingredients such as face mask packs, shampoo and conditioners are on the market (Aroma PLC, n.d.), and avocado oil was incorporated into a wide range of skin-care and hair-care products including hair conditioners, face creams, hand lotions and bath oils (James Bennett, 2018). However, research on skin health benefits of avocado as foods is rare. As there are plenty of evidences of the folk remedies of avocado due to its beneficial effect on the human skin and hair, avocado seems to be interesting by the cosmetic industry as well as in the food industry. We investigated the several types of natural foods capable of enhancing skin elasticity and accelerating of skin health recovery, which can in corporated with dairy products based on grass-fed farm milk containing ideal ratio of ω-6/ω-3 fatty acids. The purpose of this study is to determine the effects of avocado yoghurt, three beans cookie and tiger nut Galsu drink to the skin health of women in pregnancy or lactation.
Materials and Methods
The cows’ milk was provided by a dairy farm, at which 90 Holstein cows were milking. The cows were supplied according to the farm feeding system with concentrates and total mix ration (TMR). A batch of 120 L of evening milks collected and transferred to farm dairy. The raw milk was heated to 85°C for 5 min in a yoghurt ferment tank and then cooled down to 38°C for curdling the milk with the starter culture (YF-083, SACCO, Italy). The diced type of frozen avocado was purchased from the Korean grocery market and added to the separated cream for making avocado butter or cooked for making avocado jam. These avocado jam and butter were blended with the yoghurt curd after cooling down to 10°C.
Four commercial skin care foods; Biotona skin food (Keypham BVBA, Belgium), Collagen powder (Vita-Hair&Beauty, UK), Clear skin powder (As Nature Intended, UK), were purchased at natural/functional food shops in EU. Three commercial skin care drinks; BioLys (Filomediaca, Cyprus) and Happy Mama (Keypham BVBA, Belgium) which support for production of breast milk were purchased in EU. Two food formula powdered for nutrition balance and breast-feeding of lactating mother: Enfamama (Mead Johnson Nutrition, USA) and Prenagen (Kalbe Farma, Indonesia) were purchased from the market in Southern East Asia (Table 1).
A gas chromatography (HP7890, Hewlett Packard Co., USA) equipped with an FID detector, a capillary column (SP-2560; 100 m×0.25 mm×0.2 μm) was employed for the analysis of fatty acid compositions of milk and butter. Samples for injection were prepared by the official method of Korea FDA (2017; www.mfds.go.kr). The N2 gas used as carrier gas at a flow rate of 1.80 mL/min. Inlet temperature was 225°C. The oven temperature was programed to start from 140°C for 5 min, and increased at a rate of 1.5°C/min to 200°C, and kept at 200°C for 4 min and increased again to 240°C at a rate of 3°C/min and left at 240°C for 15 min. The FID detector temperature set at 270°C–285°C. The percent ratio of FA (%) is calculated by multiply the sum of standardization of correction factor (f’1) by the percent area of each peak (PAi,s) and then divided by a total of the peak area percent; Σ(f’1×PAi,s), and then the value was then multiplied by 100%. The content of FA expressed as g/100 g FA.
The portions of extract were obtained from avocado yoghurt, tiger nut Galsu drink, three beans cookie and commercial skin care foods by mixing in a 10 folds of ethyl alcohol solution and sonicated using an ultra-sonic cleaner (Branson 5510, Marshall Instruments, USA) for 30 min and repeated 3 times. The extract of mixture was filtered through 0.22 μm filter (Millipore’s, Bedford, USA.) and removed the solvent from the filtrates. Then the concentrated extracts were lyophilized for 48 hr and kept them for the analysis.
Two human cells, Hs68 fibroblasts cell line (ATCC® CRL1635TM, Sigma-Aldrich, Manassas, VA. USA) and HaCaT keratinocytes cell line (CLS, Eppelheim, Germany) were utilized for in vitro skin recovery test.
Cell viability was measured by MTT method according to the manufacturer’s instruction. The activated cell cultures were inoculated into 100 μL/well of culture medium of the 96 wells plates and incubated for 24–48 hr. MTT solution was then added and incubated at 37°C for 2 hr in a CO2 incubator. The absorbance of formazan blue formed in the cells measured at wavelength of 420 nm using a microplate ELISA reader (Molecular Devices Inc., Sunny Vale, CA, USA).
Type I C-peptide (PIP) EIA kit (Takara Medical, Inc., Shiga, Japan) was applied for ELISA assay. All reagents in the kit and samples placed at room temperature. All solutions mixed uniformly before use, being careful to avoid creating bubbles. For immunological reaction, 100 μL of antibody-POD conjugate solution was transferred into one well, and subsequently add 20 μL of sample or standard solution. After mixing those solutions promptly, the microtiter plate was sealed with aluminum foil and allowed to incubate at 37°C for 3 hr. The well contents were discarded using suction and washed the wells 4 times with 400 μL of wash buffer. After each washing step, the microplate completely emptied by inverting and tapping lightly onto a paper towel. For substrate incubation, 100 μL of substrate solution (TMBZ) was added into each well and incubate at room temperature for 15 min. After adding 100 μL of stop solution to each well in the same order as substrate solution (TMBZ), tap the plate gently to mix. The absorbance at 450 nm was measured using a plate reader and plotted a standard curve of absorbance versus the PIP concentration.
Preparation of test samples and the standards solutions followed by the manufacturer’s instructions (Cell Biologics, Chicago, IL, USA). An aliquot of 100 μL of the standards solution was loaded into empty well of the pre-coated 96-well plate. 100 μL of a series of diluted cell was poured into the empty wells and covered the 96-well plate and incubated at 37°C for 90 min. The contents of the wells discarded and blotted the plate onto paper towels. A portion of 100 μL of biotinylated antibody working solution (1:100) was added to each well and incubated at 37°C for 1 hr. The plate was washed 5 times with 300 μL PBS wash buffer and blotted the plate onto paper towels. 1 mL of ABC working solution added to each well and incubated at 37°C for 30 min. The plate was washed 5 times with 300 μL PBS wash buffer and blotted the plate onto paper towels. TMB color developing agent pre-warmed added into each well and incubated at 37°C for 15 min and stopped the reaction by adding TMB solution. The intensity of yellow was read by measure of O.D. at 450 nm in a microplate reader. Protein concentration was calculated relative O.D by subtract reading O.D with blank O.D. and plotted the standard curve by plotting O.D. 450 (relative) of each standard solution versus the respective concentration of the standards solution.
Results
The nutrients and polyunsaturated fatty acids (PUFA) compositions of farm milk and avocado butter as ingredient for making avocado yoghurt was summarized (Table 2). Fat and protein contents are important part of yoghurt, but fatty acid composition related to the facial skin health and nutritional value of dietary foods for breast-feeding. The content of unsaturated fatty acid (UFA), the ratio of ω-6/ω-3, atherogenicity index (AI), and the ratio of linoleic acid toward α-linoleic acid (LA/ALA) were important as indicator for human disease. Avocado yoghurt can supplement the high ω-6 UFA content of avocado fruit or jams and ω-3 rich farm butter (Park et al., 2018; Lee et al., 2019). The UFA content of avocado yoghurt increased from 28.89% of raw milk to 47.13% of fatty acid composition (Table 2). This result suggests that the SFA of yoghurt decreased (Lu et al., 2009; Dreher and Devonport, 2013) and the content of PUFA slightly increased from 4.23% to 5.37% since the most abundant UFA of avocado is MUFA (71%), the addition of avocado resulted in higher PUFA content. The ratio of ω-6/ω-3 did not increase as expected as avocado jam into yoghurt curd from the raw milk; 6.31 to 7.52, which is the average level present in normal milk. Another indicator of cardio circular disease of human, atherogenicity index (AI) maintained as low index as safety level as reported 1.33–2.03 by Park et al. (2018) and 2.10 by Blasko et al. (2010). The ratio LA/ALA, as other expression for indication, was also lower (7.82) than the raw milk (9.09). Compared to the ratio of ω-6/ω-3, 6.02 of TMR butter was almost two-time higher than those of the pasture butter (2.99–3.29) (Park et al., 2018) and pasture milk (3.17) (Blasko et al., 2010), This ratio appears to be high, however because of high level of ω-6 FA derived from PUFA composition of avocado, it is not so high. Such fatty acid composition of yoghurt will be beneficial for improving nutritional health as well as skin health to breast-feeding mothers and pregnant women under weight control (Duarte et al., 2016).
Fig. 1 showed the skin recovery effects of avocado on human fibroblast cell and inhibitory effects on human keratinocyte cell, and vital effect on cell viability. Although avocado extract with alcohol shows no influence on the cell vitality of Hs68, the production of pro-collagen type I C peptide increased significantly (p<0.001) in all concentrations of avocado extracts and produced the high level (1,000 ng/mL) at the concentration of 250 μg/mL. Meanwhile, avocado fruit extracts increased the cell vitality of HaCa T cell at the concentration of 125 μg (p<0.01) to 500 μg/mL (p<0.001). The MMP-1 secretion from HaCaT cell was not suppressed by the extract. It may suggest that avocado has the skin recovery effect mainly by activating of fibroblast cells than suppressing keratinocyte cells. This effect previously demonstrated in the skin beauty foods such as Galsu drink (Jung et al., 2018), three beans cookie (Lee et al., 2018) developed for the recovery of skin beauty of pregnant and lactation women.
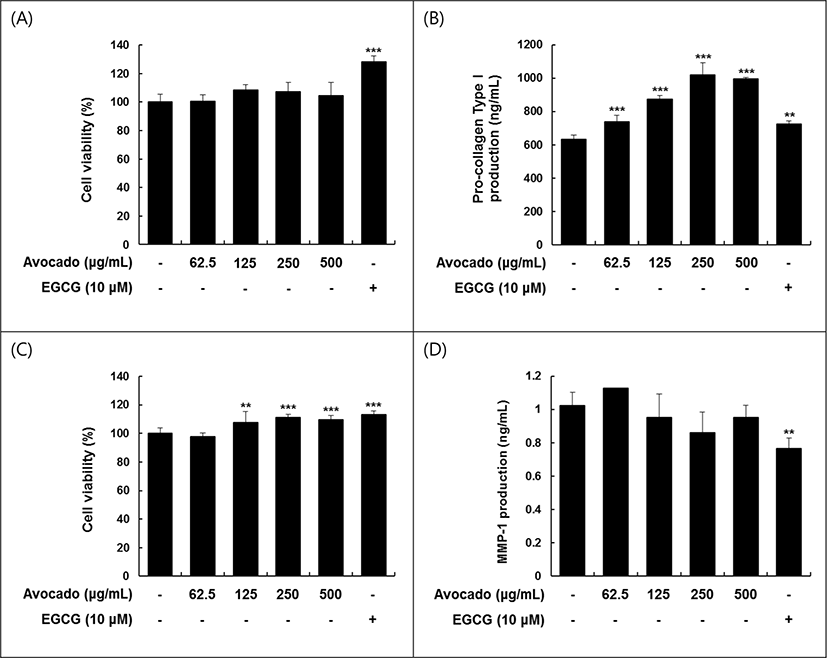
The three beans cookie are composed of black bean (Phaseolus vulgaris), lentil (Lens culinaris) and chickpea (Cicer arietinum) (Jung et al., 2018). Among the three, lentil and chickpea stimulated the cell viability in the concentration-dependent and activated the production of pro-collagen type I C peptide (125 μg/mL). However, lentil bean, black bean decreased the viability of HaCaT keratinocytes cells and lentil bean, black bean decreased significantly the production of MMP-1 secretion at the concentration of 125 μg/mL, but not as significant as chickpea (Jung et al., 2018).
The commercial skin care foods, so called “the inner health and outer beauty”, were purchased from EU natural food markets. Three of those skin care foods (Biotona skin food, Collagen powder, and Clear skin powder) were selected to test their effects on cell viability, pro-collagen type I C peptide production and MMP-I secretion. Biotona skin food (Table 3), Collagen powder (Table 4), and Clear skin powder (Table 5), did not influence on the proliferation of HS68 cells. However the proliferation of HaCaT cell by Biotona skin food has promoted at the concentration of 500 μg/mL, and Clear skin powder at 125 and 250 μg/mL (p<0.001, respectively).
The production of pro-collagen type I C peptide has accelerated only by Biotona at the concentration of 62.5 μg/mL (Table 3) and the suppression of MMP-1 secretion, but no significant changes in all concentrations.
The commercial foods support for breast milk production purchased from EU and East Asia showed significant concentration-dependent effects on the suppression of MMP-I secretion: Heath Heather (50 μg/mL, p<0.001) (Table 6), Happy Mama (50 μg/mL, p<0.01) (Table 7), BioLys (50 μg/mL, p<0.01) (Table 8). Many of botanical plant such as green tea seed extracts reported to possess the protection effect on fibroblast cell from UVB irradiation (Kim et al., 2014). The two breast-feeding support foods for pregnant and lactating mother showed high significances in Enfamama (25 μg/mL, p<0.001) (Table 9) and Pregnagen (25 μg/mL, p<0.001) (Table 10), respectively.
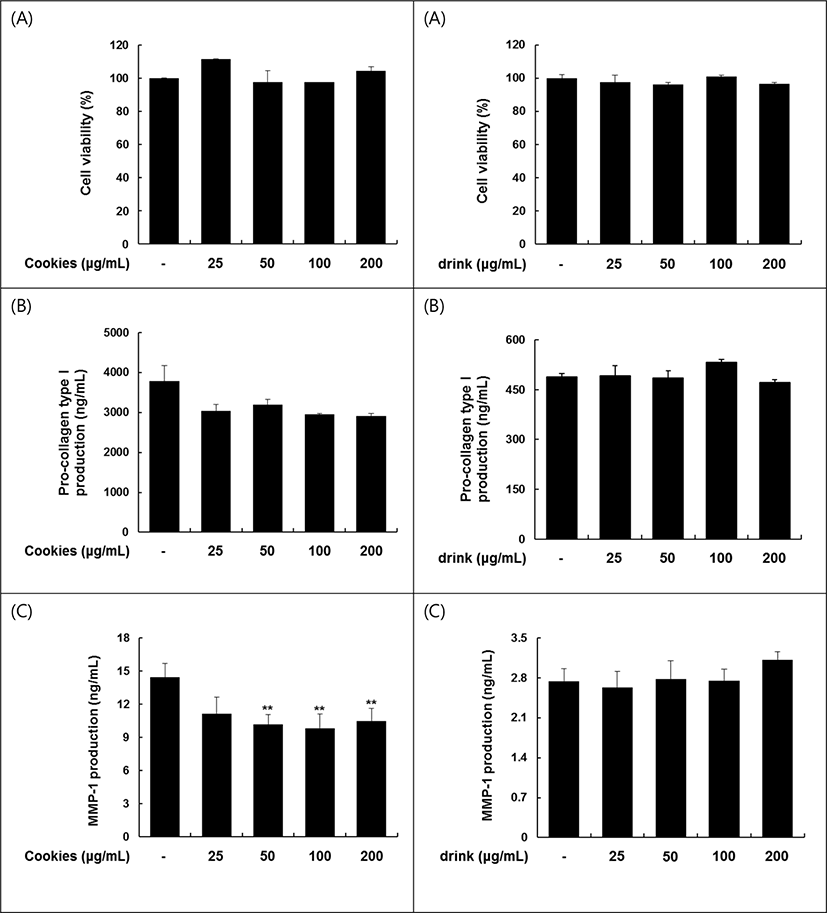
The tiger nut Galsu drink, developed by the Food Cookery Lab. of Sookmyoung Women’s University, were supplied courteously by Il-Dong Foodis Co. The tiger nut, a main ingredient of tiger nut Galsu drink reported to suppress MMP-1 secretion and release of pro-collagen type I C peptide form the two cells (Jung et al., 2018). These effects seemed to retain only in mild heat treatment condition and to lose at the commercial sterilized conditon at 121°C for 15 min. There was no skin recovery effect retained when the addition concentration has increased (Fig. 2, Right). The three beans cookie showed no dffect on the cell viability and pro-collagen type I C peptide in Hs68 cell, as well as on the suppression of MMP-I secretion (Fig. 2, Left). These results indicate that the stimulating activities of three raw beans are lost by the roasting condition. Although the ability of tiger nut showed a significant difference from the control at a minimum concentration of 62.5 μg/mL, the ability did not appear at all concentrations of Galsu drink (Table 11). The reason for significant effect of Galsu drink appears to be due to the destroy of efficacious substance by the heat treatment at high temperature and high pressure. It also assumed that the abilities of black bean, lentil, and chickpea used main ingredients of Cookie (Jung et al., 2018), were also lost in the roasting process.
Black bean, lentil, and chickpeas used for making cookie and the effects on human cell refer to the paper of Jung et al. (2018).
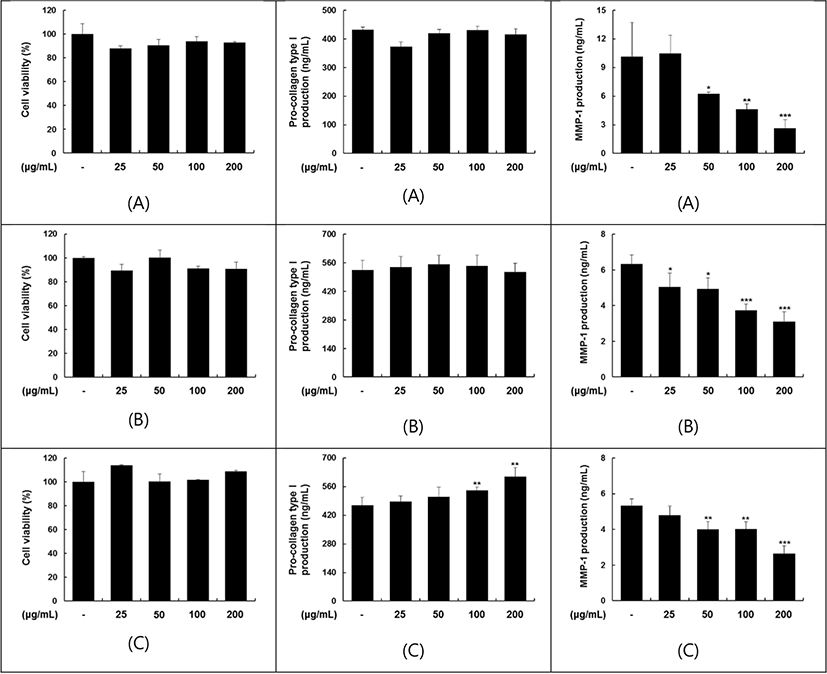
Two different recipes for avocado yoghurts and a plain yoghurt were prepared in this experiment. To the first one the frozen diced avocado added to the yoghurt base without heat treatment and the second one, a mixture of yoghurt base with avocado fruit and avocado butter and oligosaccharide.
The avocado fruit yoghurt exerted no stimulation of cell viability on Hs68 cells and showed dffect on pro-collagen type I C peptide production. On HaCaT cells revealed an effect on cell viability at the low level of concentration (62.5 μg/mL, p<0.001), but no effects on MMP-I secretion (Fig. 1). Yoghurts containing avocado fruit or fruit jam have different effects on the MMP-I secretion of Hs68 cell. Three types of yoghurt showed no stimulation effects on the cell viability, but the MMP-I secretion was suppressed at the concentration of 50 μg/mL of plain yoghurt (p<0.05) (Fig. 3A), 25 μg/mL of avocado fruit yoghurt (p<0.05), (Fig. 3B) and avocado jam yoghurt (p<0.01) (Fig. 3C), respectively. Avocado fruit promoted the production of pro-collagen type I C peptide, but the inhibition of MMP-I secretion was not observed at the maximum addition amount of 250 μg/mL, and the opposite phenomenon appeared in plain yoghurt (Table 11). This inhibition result of the MMP-1 secretion at the same level as observed in plain yoghurt indicates that the potentially efficacious substances from avocado disappeared. The effect of avocado jam yoghurt in the production of pro-collagen type I C peptide and inhibiting in secretion of MMP-1 indicates that the avocado butter, as an ingredient of avocado fruit jam, may be beneficial.
Discussion
Foods to avoid during pregnancy and breast-feeding is the most prevailing concerns, but caring their beauty is also quite intense. Instead, pregnant woman considered their infant’s health and nutrition above all. Recently breast-feeding mothers began to think more about “What nutrients do the breast-feeding mothers need ?” because what they eat determines the nutritional composition of breast milk. During the breast-feeding, the mother produces 750 mL/day of breast milk, so they need 450 kcal of energy and 25 g protein/day more than those of diets of ordinary women (Dewey, 1997). We have tried to find appropriate ingredients capable of enhancing skin elasticity and accelerating of skin health recovery by supplying nutritionally balanced foods such as dairy products based on grass-fed farm milk. Containing ideal ratio of ω-3 vsω-6 fatty acids is important. It has also recommended the consumption of one-half avocado fruit, which provides for a nutrient as well as phytochemicals (Dreher and Devonport, 2013), as healthy diet for adults and to those who need to prevent from the risk of heart diseases (Fulgoni III et al., 2013). Avocado also contains a variety of vitamins (vitamin C 2.02 mg; vitamin E 1.25 mg, carotenoids, folate, etc.), minerals and phytochemicals such as lutein, phenolic antioxidants (64 mg GAE), and phytosterols associated with numerous potential health benefits (Wu et al., 2004; Unlu et al., 2005; McCormack et al., 2010; New Zealand Avocado Consumer, n.d.). Folate, mono-saturated fats, and lipid-soluble antioxidants of avocado are good sources for maternal nutrition, which are play important roles in fertility, fetal development, birth outcomes and breast milk composition (New Zealand Avocado Consumer, n.d.). The clinical studies for health promoting effects of avocado were performed by many researchers. Grant (1960) studied on the serum cholesterol and Colquhoun et al. (1992) on the lipoproteins and apo-lipoproteins. Alvizouri-Munoz et al. (1992) and Carranza et al. (1995) investigated on plasma lipid levels and the level of blood lipids in patients with phenotype II and IV dyslipidemias. Lopez-Ledesma et al. (1996) and Carranza-Madrigal et al. (1997) studied on hypercholesterolemia. Pieterse et al. (2005) studied on the effect of avocado on weight loss, serum lipids, fibrinogen, and vascular function. Fulgoni III et al. (2013) investigated the relationships between avocado consumption and overall diet quality, energy and nutrient intakes, physiological indicators of health, and risk of metabolic syndrome from 347 consumers and 17,227 non-consumers during 2001–2008.
Hass avocado (Persea Americana) also used for making a compound butter that slab of butter placed on top of grilled foods. Although the FA composition in butter fat were very similar to those in milk fat from which butters produced, the PUFA composition of avocado butter was apparently influenced by the PUFA profile of avocado oil (Table 2). Blasko et al. (2010) reported that oleic acid (C18:1) was the most abundant (42.14%–59.19%) and followed by 18.14%–21.45% of palmitic acid (C16:0), 8.45%–15.83% of palmitoleic acid (C16:1), and 11.60%–15.14% of linoleic acid (C18:2) (Carvallo et al., 2015). In relation to health-beneficial FA and health-risk FA, summer milk and butter contained higher content of CLAc9,t11, transvaccenic acid, α-linoleic acid linoleic acid, and oleic acid, but lower content in palmitic and myristic acids, which are known as health-risk FA (Blasko et al., 2010). Meanwhile, different index of FA: ratios of oleic/palmitoleic, linoleic/palmitoleic, ratio of oleic/linoleic, LA/ALA, AI, etc. are considered as criteria of health promoting effect. The higher ratio of oleic acid to linoleic acid related to the health benefits through not only reducing the blood pressure (Teres et al., 2008) and LDL cholesterol levels (O’Byrne et al., 1997), but also reducing of type II diabetes (Vassiliou et al., 2009).
Among the nutraceutical products such as bioactive peptides, plant origin polyphenol, polyunsaturated fatty acids (PUFA), and carotenoids, their health benefits concentrated to cardiovascular and inflammatory disease; however, some studies extended their benefits to over the skin health in human trials (Perez-Sacchez et al., 2018). Roberts et al. (2009) reported that skin damage from UV or visible radiation can be protected by avocado intake, in which highly bioavailable lutein and zeaxanthin contains. Because the avocado butter made from the pasture milk contained the higher ratio of ω-3 UFA to ω-6 UFA than that of TMR milk (Park et al., 2018) and higher amount of PUFA (Table 2), the PUFA composition of avocado jam yoghurt may affect not only on the skin health but also on nutrition health of women. Stephens et al. (2016) evaluated an oral anti-aging skin supplement using a bioinstrument and self-assessments that overall facial appearance as well as mottled pigmentation and periocular wrinkles in the group of skin care supplement have changed significantly after 16 weeks. They presumed the main cause-effect related to the activation of gastrointestinal movements, but, the nutritional quality of avocado yoghurt would be a main reason in our clinical trials (data not shown). Although a lot of cosmetic skin care foods are being sold to enhance skin health, for attaining inner health and outer beauty, the skin care foods in combination with the nutrition supports for breast milk production are gradually in need.
Conclusion
The skin care foods and the foods that support breast-milk production for pregnant and lactating women are not easy to find in the natural food market in Korea than in EU and Southern East Asia. Skin care food enriched with high energy and high protein such as dairy product is essential to meet all their nutritional requirements for their beauty health and for supporting breast milk production. We may conclude that avocado yoghurt can realize the endless desire of the mothers to keep or retrieve skin beauty as well as their infant’s nutrition and health.
