Introduction
The demand for healthy food has risen steadfastly in the developed world including Korea, as household earning increases. Ginseng and red ginseng are the most popular functional foods in Korea. The root of fresh ginseng is processed into red ginseng through repeated steaming and drying (Lee et al., 2015). They have been described to enhance body’s resistance against external stress and to maintain homeostasis and traditionally used in oriental medicine (Liu and Xiao, 1992). The efficacies of red ginseng as a health functional food have been certified with the enforcement of the Korean Health Functional Foods Act in 2004. Claims for the improvement of immunity, memory, blood circulation, and menopausal women’s symptom, fatigue relief, and anti-oxidation (Kim et al., 2018; So et al., 2018) have been approved by the Ministry of Food and Drug Safety of Korea (2016).
Ginseng contains saponins called ginsenosides, nitrogenous compounds including proteins, peptides, and alkaloids, a fat-soluble component called polyacetylene, polysaccharides, flavonoids, and fatty acids (Lee et al., 2015; So et al., 2018). Ginsenosides are a class of pharmacologically active components in ginseng. Major ginsenosides are converted to minor ginsenosides with better bioavailabilty and cellular uptake by steaming to manufacture red ginseng and by treating with microorganisms and enzymes (Park et al., 2010; Jung et al., 2017).
Yogurt is another popular healthy food in Korea. Basically, yogurt can be classified into two groups, which are standard yogurt and probiotic yogurt (Fazilah et al., 2018). Standard yogurt is typically manufactured from the conventional starter culture strains, e.g. Streptococcus thermophiles and Lactobacillus delbrueckii subsp. bulgaricus. Meanwhile, probiotic yogurt is additionally supplemented with probiotic strains, such as Bifidobacterium and Lactobacillus acidophilus that are claimed certain health benefits. It has been shown that consumption of yogurt may improve lactose malabsorption, constipation, diabetes mellitus symptom, serum cholesterol level, and oral health and treat infectious diarrhea, (Fernandez et al., 2017; Pei et al., 2017; Kok and Hutkins, 2018). However, probiotic cultures cannot exert beneficial effects unless their population reaches a certain minimum level. For instances, the international standard of the International Dairy Federation suggests 107 CFU/g Lactobacillus acidophilus and 106 CFU/g Bifidobacterium in fermented milk (IDF, 1992). In Japan, the ‘Fermented Milk and Lactic Acid Bacteria Association’ has developed a standard that requires a minimum of 107 CFU/mL probiotic cells to be present in dairy products, whereas the Swiss Food Regulation as well as the Standard FIL/IDF requires that such products contain more than 106 CFU/mL (Mohammadi and Mortazavian, 2011). In contrast, Scouboutakos et al. (2017) reported that the probiotic dosages in most yogurt products sold in Canada are too low to provide the benefits shown clinical trials. They argued that further researches on probiotic yogurt are needed to promote its probiotics level (Scouboutakos et al., 2017). In order to stimulate and maintain growth and activity of probiotics in the intestine as well as in the yogurt during production and storage, probiotic yogurts are often fortified with prebiotics such as oligosaccharides, phytochemicals, and adjuvants such as protein hydrolysate, cysteine, and ascorbic acid (Shobharani and Agrawal, 2009; Mohammadi and Mortazavian, 2011; Fazilah et al., 2018).
Consuming yogurt with fruits, vegetables, whole grains, and herbs would assist in increasing intake of nutrients, prebiotics, antioxidants, phytochemicals and dietary fiber and has protective effects against specific diet-related diseases such as diabetes (Fernandez and Marette, 2017). Yogurt supplemented with various products of ginseng and red ginseng has been evaluated for its physicochemical, microbial, and sensory properties by many research groups mainly in Korea (Jung et al., 2017). The research papers showed that addition of ginseng and red ginseng to yogurt base stimulated growth of lactic acid bacteria and enhanced acid production during fermentation and viability of the bacteria during storage of yogurt (Lee and Paek, 2003; Kim and Han, 2005; Bae and Nam, 2006; Kim et al., 2008; Cimo et al., 2013; Hekmat et al., 2013; Jung et al., 2016; Jang et al., 2018).
The objective of this study is to determine the effects of supplementation of probiotic yogurt with the enzyme-bioconverted ginseng enriched with ginsenoside Rh2 (Kim et al., 2016; Lee et al., 2016), ascorbic acid, and yeast extract on viability during storage of probiotic strains in commercial starter culture.
Materials and Methods
The enzyme-bioconverted ginseng which was prepared following the modified procedure described by Lee et al. (2016) was obtained from MICO Co. (Korea). The preparation procedure is as follows. Fresh ginseng (Panax ginseng) roots of 6 years old was steamed for 2 hr at 100°C and dried for 48 hr at 80°C. The dried ginseng roots are crushed into powder and filtered using 20 meshes. 10% ginseng suspension was heated at 90°C for 2 hr and incubated with enzymes (0.9% lactase and 0.1% b-glucosidase per ginseng) at 40°C for 24 hr. The enzyme-bioconverted ginseng (EBG) powder was attained by drying the enzyme-treated ginseng suspension and then by filtering using 20 meshes.
The yogurt mix was made by adding 9.5 g of skim milk powder (Seoul Milk Co-op., Korea) to 170.5 g of commercial ultrapasteurized whole milk (Seoul Milk Co-op., Korea). Other additives, such as 0.5% enzyme-bioconverted ginseng powder, 0.025% ascorbic acid and 0.5% yeast extract (Difco, USA) were also added to yogurt mix, if necessary. The yogurt mix was made up to 200 g by adding distilled water, heated to 85°C for 1 hour, cooled to 40°C, supplemented with 0.8 mL of 1% ABT-4 (Chr. Hansen, Denmark) in the milk, and then incubated at 40°C, until pH of the yogurt mix reached 4.3. The yogurt was cooled to 4°C in ice water and then stored overnight at refrigerator. 50 g of 30% cane sugar solution which had been heated at 85°C for 1 hr and cooled to 4°C was mixed into the yogurt. The yogurt is split into 40 mL portion in the conical plastic tube and stored 4°C. The bacterial count and pH of yogurt was determined after storage for 1, 15, and 30 day. The color and viscosity were determined after storage for 3 day.
The diluent used for serial tenfold dilution of the yogurt was 0.1% peptone water. Appropriate agar plates which was pre-dried at 37°C overnight was spread with 0.1 mL of the diluted sample and incubated for colony growth. Streptococcus thermophiles was counted by using M17 agar (Difco, USA) supplemented with 0.5% lactose and incubating under aerobic condition at 37°C for 48 hours. Lactobacillus acidophilus and Bifidobacterium was counted by using BA-sorbitol and MRS-NNLP, respectively, and incubating at 37 for 72 hours under aerobic and anaerobic conditions, respectively, as described by Tharmaraj and Shah (2003). The diluent and agars was sterilized by autoclaving at 121°C for 15 min.
Forty mL of yogurt was poured on the disposable plastic petri dish with the diameter of 9 cm. The petri dish was placed on the light projection tube of measuring head of chroma meter (CR-400, Konica Minolta, Japan) to measure color. The white calibration plate was used to calibrate the meter every time before the measurements. The absolute values, e.g. L, a, and b, of Hunter Lab were measured.
Forty mL of yogurt was added into a conical tube of 50 mL volume and stored at 4°C for 3 days. Just before viscosity measurement, yogurt was incubated at 20°C for 30 min. Viscometer (TV2TLVTJO, Brookfield, USA) installed with LVT spindle No. 4 was used to measure viscosity of yogurt after the spindle turned at 10 rpm for 1 min.
Results and Discussion
The ABT-4 culture used in this study consists of two probiotic strains, Bifidobacterium BB-12 and L. acidophilus LA-5 and a starter culture, S. thermophilus. In this study, the yogurt mix after ABT-4 addition was incubated at 40°C to promote growth of the probiotic strains instead of 45°C. And the yogurt mix was heated at 85°C for 1 hr instead of 30 min, because fermentation of the yogurt mix was often delayed, when it was heated for 30 min.
Thus, the yogurt mixes which were supplemented with 0.5% EBG only and with 0.025% ascorbic acid and/or 0.5% yeast in addition to the EBG and the control yogurt mix without any supplementation were heated at 85°C for 1 hr and then fermented at 40°C, until pH reached 4.35, and then sugar solution was added. The resultant yogurts was stored at 4°C for 30 days. The yogurts were manufactured in duplicate in all the experiments.
The incubation times for all the yogurts to reach pH 4.34-4.35 ranged 7-7.5 hr. Fig. 1 shows changes of pH of the yogurts supplemented with EBG, ascorbic acid, and yeast extract during storage at 4°C. The yogurts supplemented with yeast extract in addition to EBG only and in addition to EBG and ascorbic acid showed steep pH decrease to 4.19 and 4.25, respectively, during storage for 15 day. The control yogurt and the yogurt supplemented with EBG only showed mild pH decrease to 4.32 and 4.34, respectively, during storage for 15 day. However, the pHs of all the yogurts ranged 4.15~4.21 after 30 day. These results suggested that yeast extract induced strong post-acidification during initial 15 day and regardless of supplementations all the yogurts underwent various post-acidification during 30 day.
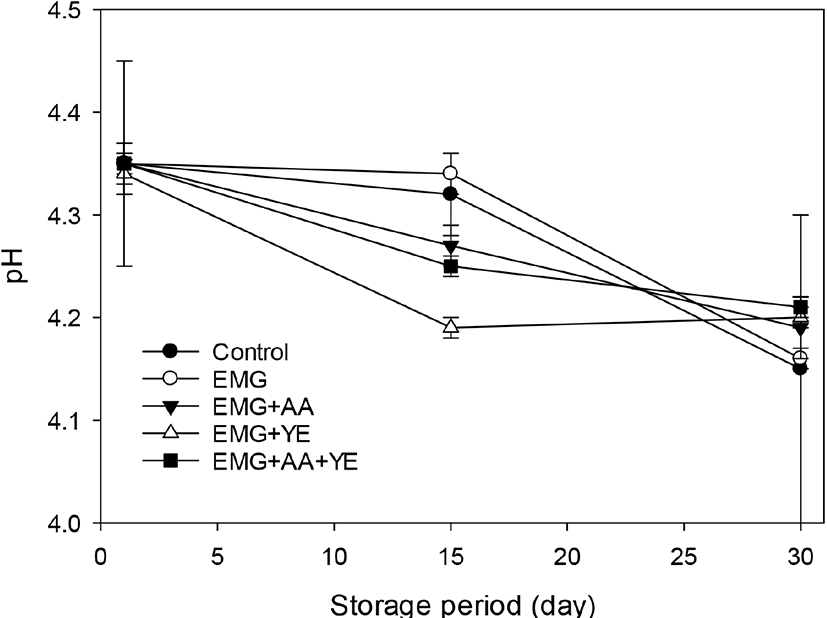
Fig. 2 shows that S. thermophilus counts of all the yogurts were more than 9 log CFU/mL and there was no decrease during storage. There was no difference in S. thermophilus counts between the control yogurt and all the other yogurts supplemented with EBG, ascorbic acid, and yeast extract. These results showed that the dominant bacterial strain in the yogurts was S. thermophilus and EBG, ascorbic acid, and yeast extract did not affect the growth of S. thermophiles and acid production during fermentation. It seems that the S. thermophilus strain in the ABT-4 culture was a rugged starter strain which did not require additional growth factors which milk could not provide.
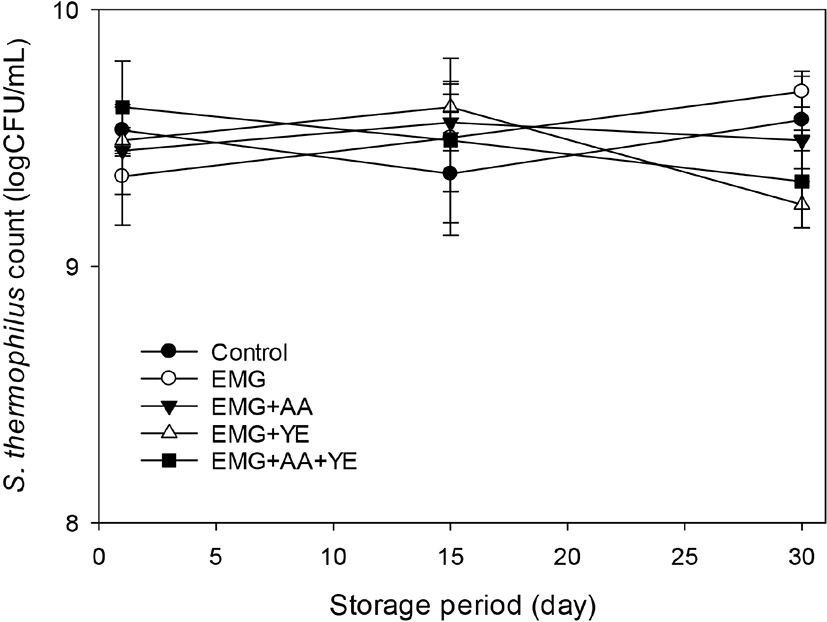
Fig. 3 shows that L. acidophilus counts of the yogurts were about 7 log CFU/mL after 1 day, but decreased to 5~6 log CFU/mL after 30 day. All the supplemented yogurts showed higher L. acidophilus count than the control throughout storage. The yogurts supplemented with EBG only and both EBG and ascorbic acid contained more than 6 log CFU/mL L. acidophilus after 30 day, but the control yogurt and the yogurts supplemented additionally with yeast extract contained less than 6 log CFU/mL L. acidophilus. These results suggested that EBG did not much promote growth of L. acidophilus, but it prevented drastic decrease of L. acidophilus count during 30 day of storage, considering the fact that the yogurt supplemented with EBG only showed higher L. acidophilus count than the control after 30 day. Supplementation with ascorbic acid in addition to EBG did not promote the growth and have additional effects on the viability of L. acidophilus. Yeast extract promoted growth of L. acidophilus during fermentation, but decreased viability of L. acidophilus during storage.
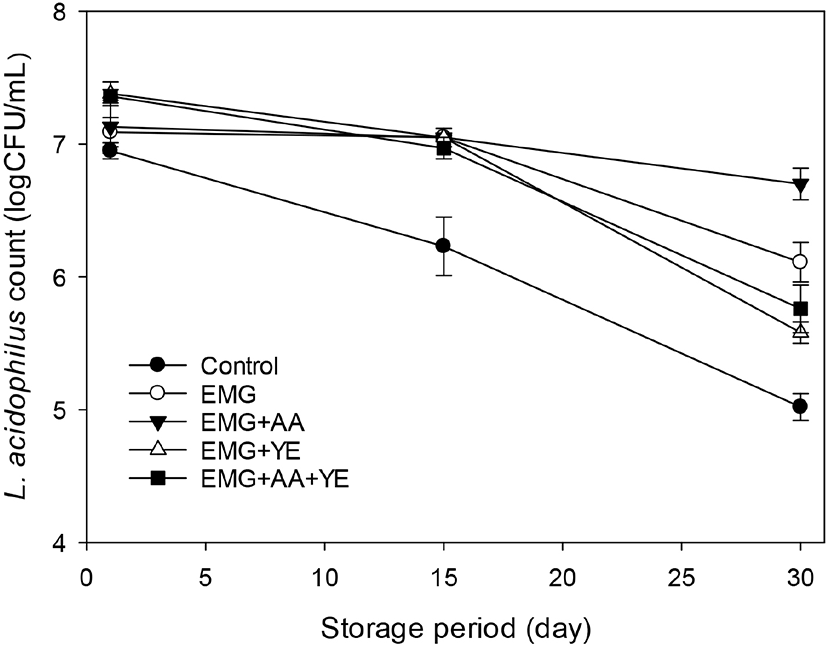
Fig. 4 shows that Bifidobacterium counts of the control yogurt and the yogurt with EBG only were 7.21±0.03 and 7.35±0.16 log CFU/mL after 1 day. The Bifidobacterium counts of the control yogurts decreased to just below 7 log CFU/mL after 30 day during storage. However, there was no decrease of the Bifidobacterium count of the yogurt supplemented with EBG only during storage. The Bifidobacterium counts of the yogurts supplemented with ascorbic acid and yeast extract in addition to EBG ranged 8.05~8.75 log CFU/mL and then decreased to 7.55~7.85 log CFU/mL after 30 day.
These results suggested that EBG did not promote growth of Bifidobacterium but maintained its viability during 30 day of storage. Ascorbic acid and yeast extract promoted growth of Bifidobacterium. The Bifidobacterium counts of the yogurts supplemented with ascorbic acid and yeast in addition to EBG were still higher than those of the control yogurt and the yogurt supplemented with EBG only after 30 day of storage. Because all the yogurt was supplemented with ascorbic acid and yeast extraction in addition to EBG in this study, ascorbic acid and yeast extract may not show their own effects on bacterial counts of yogurt during storage. It seemed that EBG, ascorbic acid, and yeast extract may interact each other synergistically to promote the growth of the probiotic strains during fermentation and to maintain viability of the probiotic strains. These results suggested that additional fortification with ascorbic acid and yeast extract of the yogurt supplemented with EBG may be advantageous to manufacture a probiotic yogurt with high count of L. acidophilus and Bifidobacterium.
The effects of different concentrations of EBG on the bacterial counts of S. thermophilus, L. acidophilus, and Bifidobacterium in the yogurts supplemented with ascorbic acid and yeast extract and the control yogurt without any supplementation were shown in Fig. 5. The yogurt mixes which were supplemented with 0.25%, 0.5%, and 1.0% EBG in addition to 0.025% ascorbic acid and 0.5% yeast extract and the control yogurt mix without any supplementation were heated at 85°C for 1hr and then fermented at 40°C, until its pH reached 4.3, and then sugar solution was added before storage of the resultant yogurt as described in Materials and Methods.
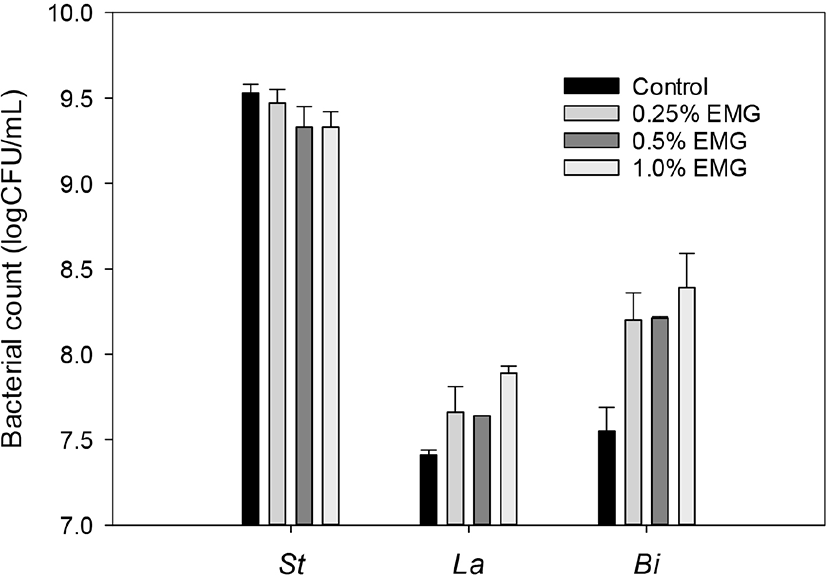
Fig. 5 showed that all the supplemented yogurts showed less S. thermophilus count, higher L. acidophilus count, and higher Bifidobacterium count than the control yogurt. As the EBG concentrations in yogurt mixes increased from 0.25% to 1.0%, S. thermophilus counts decreased slightly from 9.47 to 9.33 log CFU/mL, L. acidophilus counts increased slightly from 7.66 to 7.89 log CFU/mL, and Bifidobacterium counts increased slightly from 8.20 to 8.39. These results showed that supplementation with EBG, ascorbic acid, and yeast extract promoted growth of the probiotic strains, but additional fortification with EBG of the yogurt mix did not much improve the growth of the probiotic strains. Considering high cost to manufacture of EBG-supplemented yogurt, supplementation with lower than 0.25% EBG in addition to 0.025% ascorbic acid and 0.5% yeast extract may be feasible for commercial distribution of healthy yogurt with high probiotics counts.
The effects of different concentrations of EBG on the L, a, and b of Hunter Lab and viscosity of the yogurts supplemented with ascorbic acid and yeast extract and the control yogurt without any supplementation were investigated. Table 1 showed that as more EBG was added to yogurt, L decreased and a and b increased indicating that the color of the yogurts became deeper brown. Since ginseng root was steamed and dried, and the suspension of heated ginseng powder was heated before enzyme treatment and then dried at high temperature to manufacture EBG powder, nonenzymatic browning should have occurred to make the color of EBG dark brown. Table 1 also show that viscosity of the yogurt to which 0.25 % EBG was added was the highest, but there were little differences among other yogurts.
Several studies showed that supplementation of yogurt with ginseng resulted in high viable cell counts of bacteria (Kim and Han, 2005; Hekmat et al., 2013). When yogurts containing 1~3% ginseng were fermented with B. minimum KK-1 and B. cholerium KK-2, increase of the ginseng concentration resulted in increase of viable bacterial counts of yogurt (Kim and Han, 2005). Addition of ginseng extract to yogurt enabled L. rhamnosus GR-1 to maintain viability during storage for 28 days (Hekmat et al., 2013). American ginseng root also improved viability of L. rhamnosus GR-1 in yogurt (Cimo et al., 2013). Red ginseng extract in yogurt also promoted growth of L. acidophilus KCTC 3150 and L. salivarius subsp. salivarius CNU 27 (Bae and Nam, 2006). The EBG used in this study has also been shown to promote viability of L. acidophilus LA-5 and Bifidobacterium BB-12 during both fermentation and storage.
Ascorbic acid, when added to yogurts, can act as an oxygen scavenger and be useful to lower oxidation-reduction potential necessary to the viability of probiotic bacteria (Talwalkar and Kailasapathy, 2004). Incorporation of ascorbic acid in yogurt caused reduction in its oxygen content and redox potential during 15 and 20 days of storage (Dave and Shah, 1997). Bifidobacteria counts in the yogurt with ascorbic acid remained unaffected, whereas L. acidophilus counts decreased less rapidly. This study also showed that supplementation with ascorbic acid in addition to EBG enabled Bifidobacterium BB-12 to improve growth and viability and thus to maintain the high bacterial counts throughout storage. Ascorbic acid supplementation in addition to EBG slowed decline of L. acidophilus LA-5 in yogurt during storage.
Yeast extract is a rich source of nutrients, such as amino acids, vitamins, bases, minerals, and unknown growth factors required for optimum growth of nutritionally fastidious bacteria, such as probiotic lactic acid bacteria and can be often used as a bacterial medium components to enrich the culture. Cow milk as a growth medium for lactic acid bacteria can supply the various nutrients, including amino acid and vitamins. However, cow milk is a deficient source of bases and normally contains 80 mg/mL of orotic acid only (Robinson et al., 1983), which is a precursor in biosynthesis of pyrimidines (Loffler et al., 2016). This study showed that additional supplementation with yeast extract of the yogurt containing EBG did not shorten incubation time for acidification of yogurts, suggesting that S. thermophiles did not need yeast extract for promoting acid production in milk. However, supplementation with yeast extract increased growth of L. acidophilus LA-5 and Bifidobacterium during fermentation, but did not maintain the high bacterial count during storage, even though ascorbic acid was added together.
The major ginsenosides in EBG were shown to consist of Rh1, Rg3, Rh2, and compound K which are minor ginsenosides in fresh ginseng root (Lee et al., 2016). Lee et al. (2016) reported that the EBG treatment for 4 weeks decreased fatigue severity in healthy adult population. Chronic fatigue in a forced exercise mouse model was ameliorated by feeding Rh2-enriched Korean ginseng (Kim et al. 2016). Supplementation of yogurt with EBG may enhance synergistically the health-promoting function of yogurt by using positive interaction between probiotics and EBG.
