Introduction
Kefir was a self-carbonated alcoholic fermented milk, originating from the Caucasian Mountains (Garrote et al., 1997), and could be made from any type of processed or raw milk such as cows’, goats’or sheep’s milk (Cais-Sokolińska et al., 2008). Kefir was made through fermentation of milk using kefir grains and was a traditional beverage from Central Asia and Eastern European.
Recently, kefir has increasingly popularity around the world as new beneficent beverage (Jeong et al., 2017). The main difference between kefir and other fermented milks was the starter culture. According to the bacteria types, kefir grains contained Lactobacillus,Lactococcus, Leuconostocand Acetobacterwith two major bacteria Streptococcus thermophilus and Lactococcuslactisconsisting about 53-65% of the total microflora. Also kefir included Kluyveromyces, Saccharomyces,Candidaand Torulopsisgenus which could help to digest lactose so as to reduce lactose intolerance (Turani et al., 2014). Until now, various benefits for kefir in nutrition and health have been reported through previous studies.
Now, dietary fiber from new sources to use in food industry as a source of prebiotic has being investigated by many scientists. In general, non- digestible polysaccharides such as galacto-oligosaccharides, fructooligo-saccharides and cyclodextrins were recognized as prebiotic substances (Patel and Goyal, 2012). It could selectively stimulate the growth and activity of the gastrointestinal micro-flora, simultaneously (Lin et al., 2011) Among them, flaxseed (Linum usitatissimumL.) was the seed from the plant and could be consumed as food. Health benefits claims of flaxseed were related to its components such as lignans, α- linolenic acid, and soluble dietary fiber or mucilage/gum. According to various previous researches, the consumption of flaxseed showed several health benefits to prevent colon cancer and to reduce the risk of cardiovascular disease.
In general, flaxseeds were the richest dietary source of lignan precursors (Fig. 1). Lignans were polyphenols found in plants and Lignan precursors were found in a wide variety of plant- based foods, including seeds, whole grains, legumes, fruit, and vegetables (Borriello et al., 1985; Rickard et al., 1996).
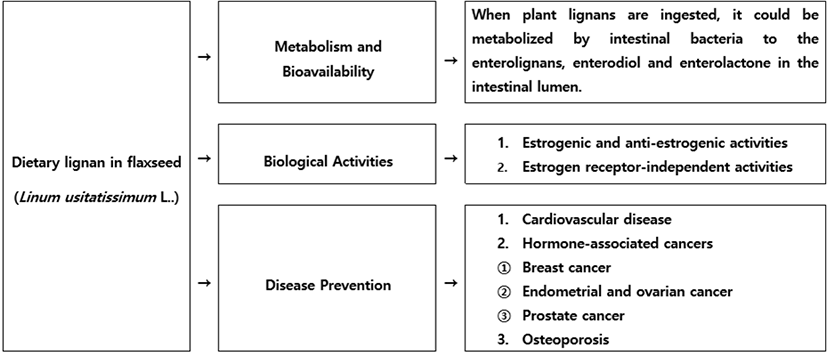
When consumed, lignan precursors were converted to the enterolignans, enterodiol and enterolactone, by bacteria that normally colonized the human intestine. Enterodiol and enterolactone had weak estrogenic activity but also could exert biological effects through nonestrogenic mechanisms (Kaur and Gupta, 2002; Westcott and Muir, 2003). Lignan- rich foods were part of a healthful dietary pattern, but further studies on the role of lignans in the prevention of hormone-associated cancers, osteoporosis, and cardiovascular disease would be needed (Fig. 2).
Besides, the heterogenic polysaccharide known as flaxseed mucilage/gum was the soluble fiber components that constituted approximately 6 to 8% of the whole seed on a dry weight basis. When flaxseed was fermented in vitro, it was resulted in producing the high amounts of acetate and propionate (short-chain fatty acids, SCFA) (Fodje et al., 2009). Also, when flaxseed mucilage used as food additives, it could capture free radicals to have anti-tumor and antioxidant properties and could oxidize proteins, lipid or DNA to prevent cancers, simultaneously (Gutiérrez et al., 2010).
Therefore, the goal of this study was to make the bioactive Kefir added with flaxseed (Linum usitatissimum L.) with upgrading the quality of organoleptic properties. In this experiment, among various physicochemical characteristics of bioactive kefir produced, TA & pH and sensory evaluation of the bioactive kefir added with flaxseed (Linum usitatissimum L.) were analyzed.
Materials and Methods
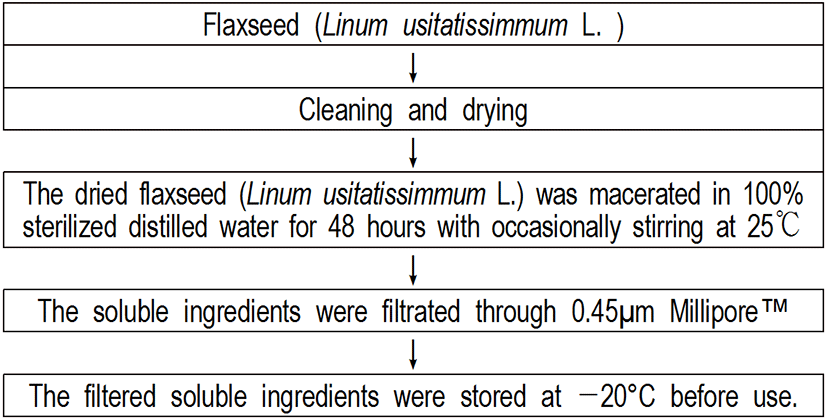
Flaxseed (Linum usitatissimum L.) was provided from Center for One Health, College of Veterinary Medicine, Konkuk University in Seoul, Korea. After cleaning and drying, the dried flaxseed (Linum usitatissimum L.) was macerated in 100% sterilized distilled water for 48 hours with occasionally stirring at 25℃. Then, the soluble ingredients were filtrated through 0.45 μm Millipore™ and stored at -20°C before use.
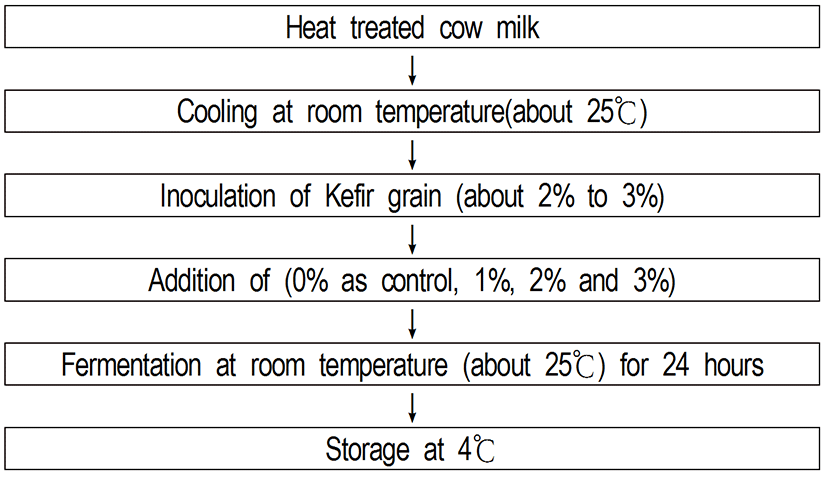
Kefir grains was obtained from Center for One Health, College of Veterinary Medicine, Konkuk University in Seoul, Korea. And kefir grain was used to ferment milk for producing bioactive kefir (Fig. 4).Bioactive Kefir samples were added with different concentration rates of flaxseed (Linum usitatissimumL..) (0% as control, 1%, 2%, and 3%). And then the bioactive kefir samples were stored at 4°C.
The pH of the homogenized yoghurt was determined using a digital pH meter (Orion Star A211, USA) and titratable acid (TA) was determined by titration with 0.1 N NaOH. Bioactive kefir sample (3 g) was transferred into an Erlenmeyer flask containing 27 mL of dH2O. Three to five drops of 0.1% phenolphthalein as pH indicator were added. The yoghurt mixture was then titrated with 0.1N NaOH with continuous stirring until as table pink color was achieved. The amount of acid produced during fermentation was calculated as follows:
Where VNaOH is the volume (mL) of 0.1 N NaOH required to neutralize the acid; a dilution factor of 10 was used.
The sensory evaluation were carried out by 10 trained panelists between 20 and 50 years of age. The samples were coded with three digit numbers and randomly served at 7 to 10℃ in plastic cups (10 mL). All assessors completed a test assessment form to compare the five sensory attributes (appearance, flavor, taste, and overall acceptability) by using a five-point hedonic scale (1, extremely poor; 2, poor; 3, fair; 4, good; 5, excellent).
Two separate experiments with duplicate assays were performed. Data were expressed as means. Statistical analysis was performed sing one-way analysis of variance (ANOVA; GraphPad Prism 5, USA) followed by Duncan’s post hoctest for mean comparison. Statistical significance was established as p<0.05.
Results and Discussion
In general, TA is high depending on the decrease of pH. In this study, the pH was decreased to about 4.51 after the fermentation of kefir premix. The pH value of bioactive kefir added with flaxseed (Linum usitatissimum L.) (1%, 2%, 3%) showed 4.50, 4,52, and 4,51, respectively. Also, the TA was increased to approximately 0.90% after the fermentation of bioactive kefir premix. The TA contents of bioactive kefir added with flaxseed (Linum usitatissimum L.) (1%, 2%, and 3%) showed 0.92%, 0.91%, 0.92%, respectively. Then, the pH and TA value between control keifr and kefir added with flaxseed (Linum usitatissimum L.) (1%, 2%, and 3%) were similar, and there was not any significant difference of TA and pH between control group and treated group.
In general, fermented dairy products were characterized by an acidic taste originating from the presence of lactic acid, a by-product of lactic fermentation. Therefore, titratable acidity (TA) and pH were commonly used as measurements of acidity to determine the quality of milk before and during the production of fermented dairy products (Ntsame Affane et al., 2009). Furthermore, the acidity of Kefir was very significant during production of kefir, because over or deficient production of acidity in kefir could have an influence to mask the buttery character and then to change the structure of the product (Vedamuthu, 2006).
When compared to previous researches, the range of pH normally reported for Kefir samples was 3.5~4.5 and the range of TA varied between 0.50 and 1.50 g 100 mL-1(Simova et al., 2002: Chen et al., 2012), and the appropriate range of pH for a commercially available yogurt was between 3.27 and 4.53, and the value of TA was in the range of 0.7% and 1.20% (Adolfsson et al., 2004).
Namely, the results of this present study showed a very similar trend with those of various previous studies (Simova et al., 2002: Ntsame Affane et al., 2011; Chen et al., 2012).
The sensory evaluation of the bioactive kefir was evaluated by 10 trained panelists of ages 20 to 50 years, and the results are summarized in Fig. 5.
The bioactive kefir was prepared with flaxseed (Linum usitatissimum L.) at concentrations of 0, 1, 2, and 3%, respectively. The overall acceptability scores of bioactive kefir with flaxseed (Linum usitatissimum L.) (0% as control, 1%, 2%, and 3%) showed 4.4, 4.5, 4.4, and 4.4, respectively. The texture scores of bioactive kefir with flaxseed (Linum usitatissimum L.) (0% as control, 1%, 2%, and 3%) showed 4.7, 4.7, 4.6, and 4.6, respectively. The colorscores of bioactive kefir with flaxseed (Linum usitatissimum L.) (0% as control, 1%, 2%, and 3%) showed 4.8, 4.8, 4.7, and 4.5, respectively. The flavor scores of bioactive kefir with flaxseed (Linum usitatissimum L.) (0% as control, 1%, 2%, and 3%) showed 3.7, 4.0, 4.2, and 4.2, respectively. And the taste scores for bioactive kefir with flaxseed (Linum usitatissimum L.) (0% as control, 1%, 2%, and 3%) showed 4.3, 4.4, 4.2, and 4.4, respectively.
Based on the statistical analysis of the sensory evaluation, there was not any significant difference of overall acceptability, texture, color, flavor, and taste between control group and treated group. Hence, among the experimental group, the scores of all categories except flavor were the same or lower in flaxseed (Linum usitatissimum L.)-containing bioactive kefir with 1%, 2%, and 3% compared with the control group. Summarizing the results of this study, the addition of flaxseed (Linum usitatissimum L.) did not affect the sensory evaluation such as overall acceptability, texture, color, and taste compared to control group without addition of flaxseed (Linum usitatissimum L.). While, the flavor showed high scores according to the addition amount of flaxseed (Linum usitatissimum L.).
According to similar previous study of Ganorkar and Jain (2014), approximately 15% incorporation level of roasted flaxseed flour in cookies resulted in acceptable product without any adversely effects on cookies. Hence, the results of this study also showed the similar trends in sensory evaluation. And color score resulted for control and different treated samples indicated that the score decreased with increasing of roasted flaxseed flour. It seems to be due to the following reasons. When flaxseed baked at high temperature, color of flaxseed could be changed from brown to dark brown (Ganorkar and Jain, 2014).Also, Pigments such as leutin/zeaxanthin in flaxseed made it dark brown, and Maillard reaction could have contributed to the darker color of bakery products due to the high protein content of flaxseed (Borrelli et al., 2003).
Next, in general, dietary fiber content could be correlated with crude fiber content. Since crude fiber content increased in roasted flaxseed flour incorporated cookies, it could be beneficial for health. Flaxseed fortification in cookies resulted in the improvement of polyunsaturated and saturated ratio while ω-6/ω-3 PUFA ratio decreased below the maximum recommended ratio. Hence, improvement in the nutritional status of roasted flaxseed flour cookies without affecting on sensory attributes establishes the suitability of flaxseed use in bakery and other food product (Ganorkar and Jain, 2014). In the results of in sensory evaluation obtained this study, similar trends were observed.
Among of many beneficial factors of flaxseed, the flaxseed lignan SECO (secoisolariciresinol) and its diglucoside SDG (SecoisolariciresinolDiglucoside) were recognized as having various benefits for health. Especially, it had the antioxidant properties. In general, after ingestion, SDG was converted to enterolignans (enterodiol and enterolactone) by the intestinal microflora, and hence these metabolites (phytoestrogens) were absorbed and could provide health benefits.
After feeding rats with specific doses of flaxseed, SDG decreased the systolic, diastolic and mean arterial pressure. SDG reduced the angiotensin I-induced rise in the arterial pressures and hence SDG was a potent Angiotensin-Converting Enzyme (ACE) inhibitor (Paschos et al., 2007; Prasad, 2009). And SDG supplementation could protect against the development of chronic diseases, cancer and diabetes on animal studies using various models of rat, mice and rabbit (Adolphe et al., 2010). Utilization of flaxseed for glycemic control would be associated to the decrease in risk of obesity and dyslipidemia, since these were risk factors for the development of diabetes and resistance to insulin (Bernacchia et al., 2014).
Flaxseed supplementation resulted in the improvement in anthropometric measurements, blood pressure, and lipid profile in the experimental group, and hence flaxseed had the therapeutic potential in dyslipidemia (Katare et al., 2012). And Saxena and Katare (2014) also reported that body mass index (BMI) and body weight of the experimental group were significantly reduced as well as systolic and diastolic blood pressure, and a highly significant reduction in total cholesterol, triglycerides, low density lipoprotein- cholesterol (LDL-C) and low density lipoproteincholesterol (VLDL-C) levels were observed, simultaneously.
Lignans could protect against certain cancers, particularly hormone-sensitive cancers such as those of the breast, endometrium and prostate, by interfering with sex hormone metabolism (Bernacchia et al., 2014). Flaxseed or pure lignans showed anticarcinogenic effects in many types of cancer, and flaxseed oil could inhibit the growth and development of tumors in the breast of laboratory animals (Lamblin et al., 2008). The influence of flaxseed lignans and oil components in reducing breast cancer risk and tumor growth was reviewed (Mason and Thompson, 2014).
Mechanisms included decreased cell proliferation and angiogenesis and increased apoptosis through modulation of estrogen metabolism and estrogen receptor and growth factor receptor signalling pathways. Also, flaxseed components were effective in the risk reduction and treatment of breast cancer (Mason and Thompson, 2014).
Lignans and other flaxseed compounds (ALA and fiber content) would be screened the efficacy in improving menopausal symptoms in women living with breast cancer and for potential impact on risk of breast cancer incidence or recurrence (Flower et al., 2013). They found that flax would be associated with decreased risk of breast cancer. Additionally, flax demonstrated anti-proliferative effects in breast tissue of women at risk of breast cancer, and also mortality risk could be reduced among those living with breast cancer (Flower et al., 2013).
Recently, there was scientifically proven that lignan and flaxseed oil reduced the growth of tamoxifen treated tumors by mechanisms involving signaling pathways, suggesting their potential use to aid in chemotherapy of some cancer types (Bernacchia et al., 2014). According to the effect of dietary flaxseed lignan or oil combined with tamoxifen treatment on tumor growth, SDG and flaxseed oil could reduce the growth of tamoxifen-treated tumors (Saggar et al., 2010). The dietary flaxseed could modestly lower serum levels of sex steroid hormones, especially in overweight/ obese women (Sturgeon et al., 2008). Also, lignans could control the growth and differentiation of cultured human leukemic cells, possibly by interfering with DNA, RNA and/or protein synthesis (Cardoso et al., 2012). Since lignan cytotoxicity appeared to be low on normal immune cells, lignans could exert fungistatic, cytotoxic, antiviral activities and a hormonal modulation with causing a decrease in hot flashes which were characteristic of menopause (Xu et al., 2008). The clinical case study based on the impact of flaxseed supplementation (30 g/day) on hormonal levels in a 31-year old woman with polycystic ovary syndrome was observed (Nowak et al., 2007). The flax consumption could alter circulating sex hormones and increased the urinary 2α- hydroxyestrone/16α-hydroxyestrone ratio associated with a lower risk of breast cancer. However, it is urgently needed for further research of flaxseed supplementation on various health benefits for human’s health.
Conclusively, the bioactive kefir added with 0%, 1%, 2%, and 3% of flaxseed (Linum usitatissimum L.) showed the decrease of pH but the increase of TA. Especially, the bioactive kefir containing 1~3%concentration of flaxseed (Linum usitatissimum L.) received higher scores for overall acceptability, texture, color, flavor, and taste in the sensory evaluation. Hence, further studies are needed to product various bioactive kefir with improving the efficiency of flaxseed (Linum usitatissimum L.) through synergy with human health.

