Introduction
Until now, numerous medicinal plants have been used for centuries and many cultures still rely on indigenous medicinal plants to meet their significant health care needs (Street et al., 2013; Jeong et al., 2016). Cichorium intybus L., commonly known as chicory, is a medicinally important plant in Asia and Eurasia including Africa. In general, Cichorium intybus L. (chicory) is an erect fairly woody perennial herb, about 1 m in height with a fleshy taproot of over 75 cm in length and large basal leaves (Bais and Ravishankar, 2001) (Fig. 1).

All parts of Cichorium intybus L. (chicory) have many important medicinal components such as alkaloids, inulin, sesquiterpene lactones, coumarins, vitamins, chlorophyll pigments, unsaturated sterols, flavonoids, saponins, tannins, and so on (Nandagopal and Kumari, 2007; Muthusamy et al., 2008; Atta et al., 2010). Furthermore, fresh chicory generally includes about 68% inulin, 14% sucrose, 5% cellulose, 6% protein, 4% ash, and 3% other compounds, on the other hand dried chicory has roughly 98% inulin and 2% other compounds (Meehye and Shin, 1996).
Although important phytochemicals are distributed throughout the plant, the main components are present in the root of Cichorium intybus L. (chicory) contained over 40% inulin (Bais and Ravishankar, 2001; Judzentiene and Budiene, 2008). To date, Cichorium intybus L. (chicory) is grown for the production of inulin on an industrial scale (van Arkel et al., 2012).
Also, there is a little investigated the bioavailability of Cichorium intybus L. (chicory) in terms of phytochemistry and pharmacology. In fact, up to 100 individual compounds have been isolated and identified from the roots of Cichorium intybus L. (chicory) (Street et al., 2013). Most of the pharmacological studies on Cichorium intybus L. (chicory) have been performed the testing of aqueous and/or alcoholic extracts only (Street et al., 2013).
Among various food-borne pathogenic bacteria, Bacillus cereus is a spore-forming bacterium which occurs naturally in many kinds of foods and can cause illness in humans (Jaquette and Beuchat, 1998; Agata et al., 2002). It could form spores which are resistant to heating and dehydration and therefore could survive cooking and dry storage (Paananen et al., 2002; Clavel et al., 2004). When foods containing Bacillus cereus spores are in the ‘temperature danger zone’ the spores may germinate, and the bacteria may grow, produce toxins, and make people sick (Ehling- Schulz, 2004). Such illness is frequently linked with starchy foods of plant origin such as rice, pasta, potatoes, pastry and noodles. Bacillus cereus can cause vomiting or diarrhoea and, in some cases, both (Guinebretière et al., 2008). Enterotoxins produced by B. cereus (diarrhoeal toxin) result in the diarrhoeal form of the disease and most often follow ingestion of contaminated food, local bacterial growth and subsequent toxin production in the intestines of the host (Berthold-Pluta et al., 2015). Emetic toxin produced by B. cereus (cereulide) can result in the vomiting form of the disease following ingestion of food containing pre-formed toxin (Rajkovic, 2014). Generally, diarrhoeal illness is often associated with meat products, soups, vegetables, sauces and milk/milk products. Especially, dairy products may spoil through the growth of spores (including the spores of psychrotrophs) that survive pasteurization (Rajkovic et al., 2008).
So far, there was no report on the inhibition against Bacillus cereus as food-borne pathogenic bacteria using the crude extracts from Cichorium intybus L. (chicory). Therefore, this study was aimed at investigating the antimicrobial activity of Cichorium intybus L. (chicory) as natural additives against Bacillus cereus for guaranteeing the quality and safety of various dairy products.
Materials and Methods
Cichorium intybus L. (chicory) were donated from Center for One Health, Konkuk Univeristy in Seoul, Korea. The roots of Cichorium intybus L. (chicory) were cleaning, cutting into slices, and drying. Then the dried roots were macerated in 95% ethanol for 48 hours with occasionally stirring at 25±2℃. And hence, the soluble ingredients were concentrated by rotary evaporator (RE801C-W, Yamato, JAPAN) at 50±1℃ until dryness. The yield obtained was obtained for ethanol extraction type. These stock solutions were filtrated through 0.2 mm millipore and stored at -20℃ before use.
Bacillus cereus ATCC 10876 was obtained from Department of Public Health, College of Veterinary, Konkuk University in Seoul, Korea. Cells were grown on nutrient agar (NA; Oxoid, UK) overnight. According to the instruction of the manufacturer, colonies were transferred into tubes containing cryopreservation fluid (Original Microbiology Bead Storage System, STS, Technical Service Consultants Limited, UK). Until use, the beads were stored at -70±5℃.
Antimicrobial activity of Cichorium intybus L. (chicory) was detected by the spot on lawn method with some modifications (Cadirci and Citak, 2005). All test bacteria were cultured on Mueller-Hinton broth (MHB; Difco) and incubated at 37℃ for 24 h. The culture broth was diluted using MHB to 0.5 McF and spread onto Mueller-Hinton agar (MHA; Difco) using sterilized cotton swabs. A total of 10 μL, 20 μL, and 30 μL of chicory extract was directly dropped onto the surface of the MHA, respectively. The plates were incubated for 24 h at 37±1℃, and the inhibition zone was observed.
All experiments were carried out independently in triplicate experiments. The inhibition of various concentration of Cichorium intybus L.’s (chicory) ethanol extracts against Bacillus cereus ATCC 10876 were evaluated by one way analysis of variance (ANOVA).Statistical significance was accepted at the P = 0.05 level.
Results and Discussion
In this study, the ethanol extract of Cichorium intybus L. (chicory) showed various levels of antimicrobial activity when tested by spot on lawn method (Fig. 2). This results obtained showed that ethanol extract exhibited antimicrobial activities against Bacillus cereus ATCC 10876. With increasing concentration of Cichorium intybus L.’s (chicory) ethanol extracts, the results produced the larger zones of inhibition against the bacteria tested (P<0.05) (Fig. 2).
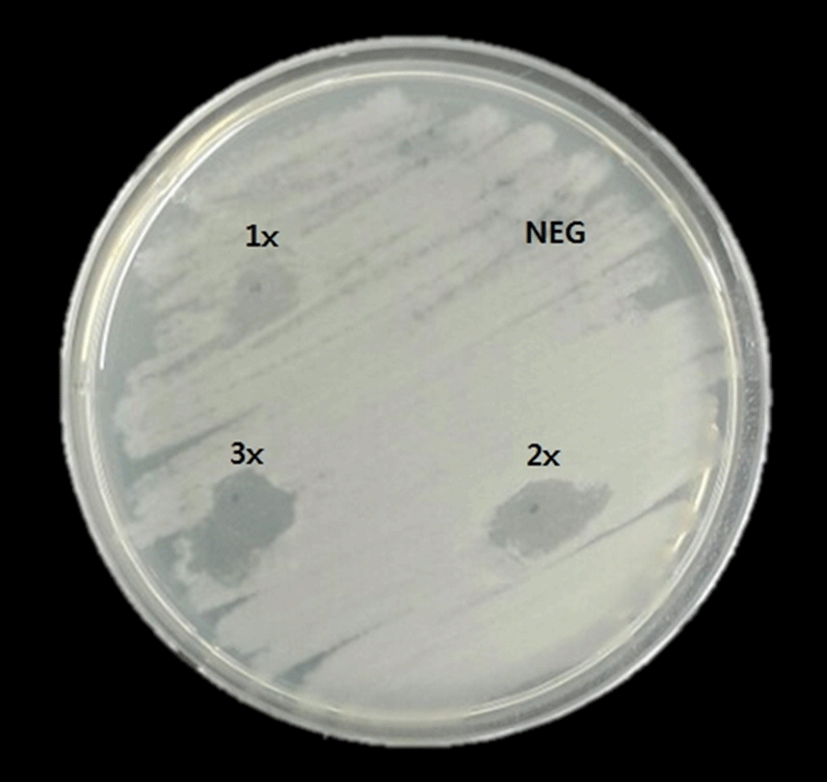
Until now, many natural materials including Cichorium intybus L. (chicory) have been studied for developing natural additives with antimicrobial activity by researchers, and also for replacing synthetic compounds (Rai and Khullar, 2004; Street et al., 2013). Furthermore, the comparative activities of Cichorium intybus L. (chicory) as natural materials were analyzed by extraction method using various solvents such as chloroform, chloromphenicol, ethyl acetate, hexane, petroleum ether, water, etc. (Petrovic et al., 2004; Nandagopal and Kumari, 2007).
For example, Petrovic et al. (2004) investigated the antimicrobial activity of the water, ethanol and ethyl acetate extracts of Cichorium intybus L. (chicory), respectively. Although all the tested extracts showed antimicrobial activity, the ethyl acetate extract corresponded to highest value. Water extract inhibited Agrobacterium radiobacter sp. tumefaciens, Erwinia carotovora, Pseudomonas fluorescens and Pseudomonas aeruginosa. Also, in comparison of leaves and roots of Cichorium intybus L. (chicory), root extracts have more intensive antibacterial activity than extracts from whole part (Petrovic et al., 2004).
According to the research of Nandagopal and Kumari (2007), they used both non-polar as well as polar solvents for extracting active components from the roots of Cichorium intybus L. (chicory). The results showed that all the five solvent extracts had antimicrobial activity against Bacillus subtilis, Staphylococcus aureus and Micrococcus luteus (gram positive) and Escherichia coli and Salmonella typhi (gram negative). Cichorium intybus L. (chicory) showed significant antimicrobial activity which is probably due to the presence of various bioactive materials such as bitter sesquiterpene lactones, coumarins, flavonoids, inulin, and so on (Nandagopal and Kumari, 2007).
Of course, the antimicrobial activity was expressed at varying degree which was being both strain and does dependent (Nandagopal and Kumari, 2007). The various crude extracts of Cichorium intybus L. (chicory) showed significant activity against all the bacteria tested (reference). Similar to this study, the biological activity of Mentha pipertia against the pathogenic bacteria was reported (Deans et al., 1998).
Furthermore, Al Akeel et al. (2014) studied that the antimicrobial activity of different plant extracts prepared in different pH and buffers was tested against different bacterial strains. Cichorium intybus L. (chicory) was found to be effective on Pseudomonas aeruginosa, Escherichia coli and Pproteus vulgaris with a zone of inhibition 10 mm, 11 mm, and 10 mm, respectively. For maintaining human health and infections control, plants including Cichorium intybus L. (chicory) have been a valuable source of natural products as antimicrobial agents (Al Akeel et al., 2014). Hence, with plant extracts have great potential as antimicrobial compounds against microorganisms, such plants including Cichorium intybus L. (chicory) should be investigated to better understand their properties, safety and efficiency (Mothana et al., 2009).
Next, Gazzani et al. (2000) reported that the antimicrobial activity of the organic acid-rich extract of fresh red chicory (Cichorium intybus var. sylvestre) was tested against periodontopathic bacteria including Streptococcus mutans, Actinomyces naeslundii, and Prevotella intermedia. The compounds identified from the active extract include oxalic acid, quinic acid, shikimic acid, succinic acid, and so on. Especially, all of the organic acids were found to decrease biofilm formation and adhesion of bacteria to the cells, with different levels of efficacy (Carazzone et al., 2013). Hence, these compounds also induced biofilm disruption and detachment of dead cells for the cultured substratum (Gazzani et al., 2000). Also, on the antimicrobial activity of Cichorium intybus L. (chicory), root extracts had pronounced effects on Bacillus subtilis, Staphylococcus aureus, Salmonella typhi, Micrococcus luteus, and Escherichia coli (Shaikh et al., 2012). And The leaf extract of C. intybus also showed a moderate activity against multidrug resistant S. typhi (Rani and Khullar, 2004). Guaianolides- rich root extracts of C. intybus have shown antifungal properties against anthropophilic fungi Trichophyton tonsurans, T. rubrum, and T. violaceum (Mares et al., 2005). A sesquiterpenoid phytoalexin cichoralexin isolated from chicory exhibited potent antifungal activity against Pseudomonas cichorii (Monde et al., 1990),
And furthermore, Cichorium intybus L. (chicory) had been reported to possess (a) Antimicrobial activity, (b) Anthelmintic activity, (c) Antimalarial activity, (d) Hepatoprotective activity, (e) Antidiabetic activity, (f) Gastroprotective activity, (g) Anti-Inflammatory activity, (h) Analgesic activity, (i) Antioxidant activity, (j) Tumor-Inhibitory activity, (k) Antiallergic activity, (l) Other Pharmacologically Important activities, and so on (Street et al., 2013; Abbas et al., 2015). And Cichorium intybus L. (chicory) is a beneficial herb and also contains high amount of proteins, carbohydrates, mineral elements, good agronomic characters and high biomass production (Wang and Cui, 2011).
In conclusion, the present study showed the antimicrobial activity of Cichorium intybus L. (chicory) to inhibit the growth of Bacillus cereus. Also Cichorium intybus L. (chicory) could be used for the natural agents as antimicrobial agents. However, further study should be undertaken so as to identify the bioactive functions of the crude extract from Cichorium intybus L. (chicory). Furthermore, it is required to guarantee the quality of the crude extract from Cichorium intybus L. (chicory) for evaluating biological activities and health-promoting properties.
