Introduction
Probiotics are microorganisms that are believed to provide health benefits when consumed (Kim et al., 2015; O'Brien et al., 2016). A probiotic is a live microbial food supplement used in fermented dairy foods and cheeses that beneficially affect the host animal by improving the microbial balance (Samah et al., 2016). In general, the probiotic could improve colonization resistance, immunomodulation and nutritional contribution of the normal gastrointestinal microbiota when ingested by human or animal hosts (Khoder et al., 2016). Furthermore, potential health benefits of probiotics claimed in the literature are substantial including inhibition of carcinogenesis and anticholesteremic effects (Rababah et al., 2015; Del Carmen et al., 2016). Namely, the improvement of digestibility is generally attributed to the presence of the enzyme lactase that is associated with kefir-isolated lactic acid bacteria and other probiotic organisms (Kim et al., 2015; Zavala et al., 2016). Recently, kefir is actually considered an example of probiotic mixture of bacteria and yeasts (Guzel-Seydim et al., 2011; Kim et al., 2015). Probiotics as lactic acid bacteria generally showed higher the lactase activities (Kim et al., 2015). Lactase was a part of the β-galactosidase family of enzymesand was a glycoside hydrolase involved in the hydrolysis of the disaccharide lactose into constituent galactose and glucose monomers (Kim et al., 2015). Until now, the lactase activity of Bifidobacterium bifium, Lactobacillus acidophillus, and a mixed yoghurt culture of Streptococcus thermophilus and L. delbrueckii ssp. bulgaricus was observed by several previous studies (Yoon et al., 2000; Shin et al., 2012; Kim et al., 2015). However, there was no report about the lactase activity of Kefir- isolated LAB until now.
Hence, the objective of this study were to examine variability of lactase activities of kefir-isolated lactic acid bacteria strains and also to evaluate methods for determining potential activities of the enzyme associated with the culture microorganisms.
Material and Methods
Lactobacillus kefiri DH5 was isolated from the kefir grains. Bifidobacterium animalis subsp. lactis and Bifidobacterium longum 720 were purchased from Sacco®-LyofastBLC1 (Sacco, Cadorago, Italy) and Culture Systems, Inc. (Mishawaka, IN, USA), respectively (Table 1). Three strains of lactic acid bacteria were grown in MRS broth (Oxoid, Basingstoke, Hampshire, UK)at 25℃ for 48 h.
Suspensions (100 mL, each) of reconstituted 10% nonfat dry milk were heat-treated at 83℃ for 30 min in a water bath and cooled to 40℃ at ambient temperature. Based on preliminary study(Kim et al., 2015), the suspensions were inoculated with approximately 108 CFU/mL at 1~2% level (wt/vol). The inoculated suspensions were fermented in a 38℃ water bath for active enzyme production by the cultures. All suspensions were prepared in duplicates for each culture.
The reference standards of lactose, glucose, galactose, and fructose (purity ≥ 99.0%, for HPLC) were purchased from Sigma– Aldrich (St. Louis, MO, USA). Deionized water was purified by a Milli-Qsystem (Milipore Corp., Bedford, MA, USA) and 1N sulfuric acidwas from Fischer Scientific (Piscataway, NJ, USA) and diluted with deionized water for use. All solvents were HPLC-grade and were used without further purification. A set of standard solutions and quality control (QC) solutions for various sugar were prepared by successive dilution of the stock solutions (1 mg/mL for each standard samples) with purified deionized water. All solutions were stored at -80℃ until used in analyses. A set of 3 lactic acid bacteria were selected to compare its lactase activity during 48 hours. One strain was isolated from kefir milk and 2 strains were food grade commercial product used to fermented products as starter culture. More details about 3 strains were described on Table 1.
According to the methods provided by Kim et al. (2015), sample analysis was performed using Agilent 1260 HPLC ChemStationTM (Agilent, Palo Alto, USA) equipped with UV Detector and RI detector (Table 2). The separation of sugars was performed on a Hi-Plex Ca column (6.5 mm ×300 mm) using an automatic injector (G1367E, 1260 Hip-ALS, Agilent Technologies 1290 Infinity) and Guard Column (4.6 mm ID ×12.5 mm, Agilent, USA). Mobile phase was 0.013M H2SO4 at a flow rate of 0.8 mL/min. The column and auto-sampler tray were maintained at 65℃ and 4℃, respectively. The analytical run time was 20 min.
Statistical analysis was carried out to compare enzyme activities in suspensions of different culture strains using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). The concentration of lactose, glucose and galactose were computed for Least-Squares means using SPSS GLM procedures, and mean concentrations were tested for significance (p<0.05) using Duncans multiple range test. The experiment was duplicated.
Results and Discussion
Kefiris a fermented milk drink made with kefir “grains” (a yeast/bacterial fermentation starter) and has its origins in the north Caucasus Mountains (Ianeva et al., 2012; Rahimzadeh et al., 2012). Kefir grains are a combination of live bacteria and yeasts that exist in a symbiotic matrix on a surface of a complex polysaccharide with a casein core (Prado et al., 2016). However, the make-up of kefir grains could vary depending on the culturing location and culturing conditions (Guzel-Seydim et al., 2011).The list of bacteria and yeast strains are Table 3.
Resource from Guzel-Seydim et al. (2011), Ianeva et al. (2012), and Ahmed et al. (2013)
Hence, among bacteria strains belong to kefir grains, kefir-isolated lactic acid bacteria should be firstly screened or evaluated by the lactase activity using HPLC, and then the selected probiotic candidate strain (s) of kefir-isolated LAB could be applied for various milk-based fermented foods with higher functional properties of probiotics, either alone or combined with other probiotics. Furthermore, among yeast strains belong to kefir grains, yeast also should be monitored so as to improve the lactase activity.
The initial fermentation of the culture suspensions resulted in considerable differences of lactose, glucose, and galactose. During the fermentation, the culture organisms not only fermented lactose but also converted it to glucose and galactose (Yoon et al., 2000; Kim et al., 2015). This is reflected on the initial concentration of lactose, glucose, and galactose in samples. Fig. 1 showed the typical high performance liquid chromatography chromatogram of 1% standard solution.
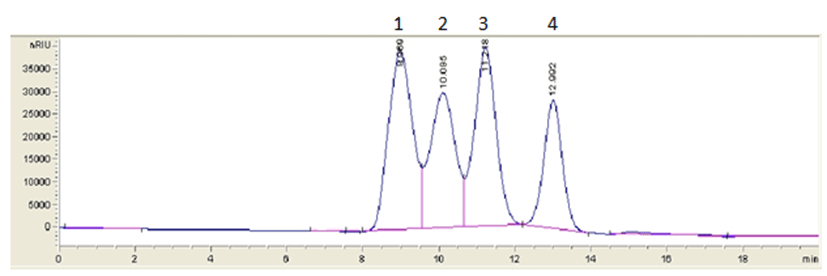
AgilentTM Hi-Plex Ca Carbohydrate Analysis column (6.5 mm×300 mm): Flow rate, 0.8 mL/min; Mobile phase, 0.013 N H2SO4; RI detector: 1. Lactose, 2. Glucose, 3. Galactose, and 4. Fructose.
The concentration of lactose in the samples decreased steadily, and the greatest reduction of lactose was observed with the BL720, followed by DH5, and BLC cultures (Fig. 2). Also, the concentration of galactose showed changes but, that of glucose showed little changes during 48 hours of incubation (Data not shown).
Based in this study, the concentration of lactose was decreased from 27% (BLC) to 36% (DH5 and BL720) (p<0.05) (Fig. 2), and the concentration of galactose was increased about 200% (DH5, BL720, and BLC) (p<0.05) (data not shown). However, The DH5, BL720, and BLC cultures test in this study showed no increase in glucose concentration during 48 hours of incubation (Data not shown).
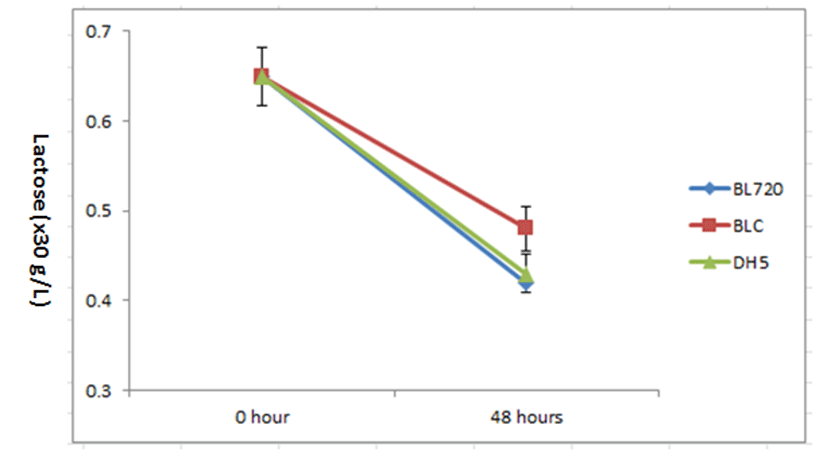
Even though sonication or antibiotic are not treated toward all 3 probiotic strains used in this study, DH5 as kefir-isolated lactic acid bacteria showed the greatest reduction of lactose and the largest increase of galactose. This study revealed that the lactase activity of DH5 showed the similar to that of Bifidobacterium spp. which was recognized as the highest activity of lactase compared to other lactic acid bacteria. Hence, for improving the usability of kefir-isolated LAB, it should be focused on screening and selecting the kefir-isolated LAB with high levels of both intra- and extra- cellular lactase using by sonication and antibiotic treatment. If above mentioned conditions are met, Kefir-isolated LAB will be important with regard to minimizing the lactose mal-digestion problem.
The formations of different various saccharides were observed in the culture strains during 48 hours incubation. The BLC culture showed the peak of oligosaccharide, tagatose and unknown peak, and the BL720 culture exhibited the peak of tagatose and unknown peak without the peak of oligosaccharide (Data not shown). However, the BLC culture exhibited the largest number of monosaccharides (tagatose and several unknown). This result showed the similar patterns to previous study (Yoon et al., 2000; Shin et al., 2012; Kim et al,, 2015). Also, there was no detection on the peak of xylose andsedoheptulose at all 3 lactic acid bacteria used in this study.
In general, the formation of oligosaccharides is a well known phenomenon during hydrolysis of lactose by lactase (Shin et al., 2012; Kim et al., 2015).The enzyme is known to take galactosyl moieties from lactose molecules and transfer them forming new oligosaccharides (Yoon et al., 2000; Liu et al., 2011; Gobinath and Prapulla, 2014; Kim et al., 2015). Especially, it is interesting that less oligosaccharides and more monosaccharides were detected in DH5 as kefir- isolated lactic acid bacteria. Since the Hi-Plex Ca Carbohydrate Analysis column used in this study was 8% crosslinked resin, saccharides should had been eluted in decreasing order of molecular sizes (Yoon et al., 2000; Kim et al., 2015). Hence, those compounds eluted earlier than lactose are most likely oligosaccharides having di- or tri-saccharide units and others saccharides present included xylose, sedoheptulose, tagatose, and so on (Splechtna et al., 2006; Shin et al., 2012; Kim et al., 2015).
Also, based on this study, all 3 lactic acid bacteria showed the peak of tagatose. The origin of tagatose seemed to be a byproduct of the metabolism of galactose to pyruvate by the BBB culture organisms (Pidoux et al., 1990; Yoon et al., 2000; Kim et al., 2015). Hence, DH5 as kefir-isolated LAB maybe have the similar characteristic and properties of Bifidobacterisum spp. such as BL720 and BLC cultures. Furthermore, a seven- carbon ketose as xylose and sedoheptulose could be produced in the metabolic pathway of Bifidobacterium spp. (Frengova et al., 2000; Shin et al., 2012; Kim et al., 2015). But all 3 lactic acid bacteria used in this study did not shown any peak of xylose and sedoheptulose. Then, the further studies are needed to assess current outcome.
In summary, total 3 strains of lactic acid bacteria which were 1 of kefir-isolated and 2 of commercial exhibited a considerable difference in their lactase activities. Incubating the culture suspensions at 37℃ was sufficient to detect significant changes in concentration of lactose, glucose or galactose by HPLC. Also further study is needed that whether sonication or antibiotic treatment could be used for differentiating intra- and extracellular lactase activities associated with kefir- isolated LAB cultures.
